Tue, 16 April 2024
In this episode, we discuss artificial intelligence large language models (LLMs) and how these will impact the future of the practice of pharmacy. Key Concepts - Generative AI with large language models (LLMs) have already changed how healthcare is delivered to patients. In the future, these changes will be more substantial and require pharmacists and other healthcare professionals to understand the benefits and downsides of this technology.
- Commercial LLMs, such as ChatGPT, are not HIPAA compliant and should not be used with protected health information. Companies currently offer software products that are HIPAA compliant and can integrate directly into electronic health records in a HIPAA-compliant manner.
- Currently, most commercial use cases of LLMs for healthcare providers focus on expediting or simplifying the documentation process (e.g. generating a first draft of a progress note or summarizing a patient encounter from an audio recording).
- In the future, LLMs will be used to perform a variety of clinical tasks, including drug interaction checking, renal dose adjustments, duplication of therapy, and even the appropriateness of a patient’s drug regimen for a given medical condition. These clinical tasks will almost certainly be done as a “first pass” to highlight or flag specific aspects of a patient’s chart and will then be reviewed by a licensed (human) healthcare provider as a final check prior to clinical decisions being made.
References - Large Language Models (LLMs) referenced in the episode: https://chat.openai.com, https://coral.cohere.com, https://claude.ai, https://gemini.google.com.
- Prompt Engineering Guide (https://www.promptingguide.ai/techniques)
- OpenAI - Prompt engineering (https://platform.openai.com/docs/guides/prompt-engineering/six-strategies-for-getting-better-results)
Direct download: 181-ai-pharmacy.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 26 March 2024
In this episode, we review the pharmacology, indications, adverse effects, monitoring, and unique drug characteristics of HMG CoA reductase inhibitors (“statins”). Key Concepts - Statins reduce LDL cholesterol by 20-60% (depending on the dose and statin potency). They have modest favorable effects on HDL and triglycerides. Clinically, statins reduce the risk of major adverse cardiac events by about 30% depending on the statin potency.
- There are four main groups of patients who are indicated for a statin: LDL >= 190 mg/dL, diabetes with age 40-75 years with LDL 70-189 mg/dL, those with an elevated 10-year ASCVD risk of > 7.5% (or possibly > 5%), and those who have had an ASCVD event (“secondary prevention”).
- Atorvastatin, lovastatin, and simvastatin heavily rely on CYP 3A4 metabolism and tend to be most susceptible to drug interactions compared to the other statins.
- When a statin is started, baseline lipid panel and liver function tests should be obtained. After 4-12 weeks, a lipid panel should be repeated. Liver function and creatine kinase testing should only be done if a patient has a symptom (e.g. jaundice, right upper quadrant pain, muscle pain or weakness, dark urine, etc.)
References - Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. https://www.ahajournals.org/doi/10.1161/CIR.0000000000000625
Direct download: 180-statin.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 5 March 2024
In this recurring episode, we discuss the important updates from the 2024 American Diabetes Association Guidelines! Key Concepts - Tirzepatide is now recommended as one of the weight loss pharmacotherapy options along with semaglutide in patients with diabetes. The language for its use in comparison to insulin therapy has been updated similar to GLP-1RAs.
- The new hypoglycemia section in chapter 6 now houses all recommendations regarding screening, education, prevention, and treatment of hypoglycemia. The recommendation for prescribing glucagon has been clarified - regardless of type of diabetes, it is recommended that glucagon be prescribed to all patients using insulin or those who are at high risk with proper education of family members or caregivers.
- Teplizumab, a monoclonal antibody against CD30, is available for preventing progression of stage 2 type 1 diabetes to stage 3 type 1 diabetes. Guidelines have updated screening criteria for staging type 1 diabetes and recommends use of teplizumab in these patients.
- Other updates revolve around emphasis of using diabetes technology such as CGMs and AID for appropriate patients, clarified or strengthened screening recommendations for type 1 staging, peripheral arterial disease, bone mass density, etc., and emphasis on weight management alongside meeting glycemic goals.
References
Direct download: 179-dm2024.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 13 February 2024
In this episode, we speak with Janeen Winnike, the Associate Dean for Student Affairs at Rosalind Franklin and a co-course director for the Pharmacy Law course at the university. We review some of the key points regarding federal and Illinois pharmacy law – a must-listen especially for graduates preparing for their MPJE exam after graduation! Key Concepts - The FDA (via the Food, Drug, and Cosmetic Act) primarily regulates manufacturers. Most regulation for pharmacies and pharmacists is via the federal Controlled Substances Act and state-based regulations (acts and administrative codes).
- An IND (investigational drug application) is required to begin human clinical trials (phase I-III). An NDA (new drug application) is used for the FDA to consider whether a drug should be approved for use in the US.
- The Federal Controlled Substances Act outlines which drugs are scheduled I-V. State law can be more restrictive. C-II drugs have special regulations related to prescribing, ordering/distribution, refills, partial fills, etc.
- In Illinois, pharmacists, student pharmacists, and pharmacy technicians are permitted to vaccinate patients aged 7 years and older (or temporarily 3 years and older per the PREP act for COVID-19 and influenza vaccines). Pharmacists can order and administer COVID-19 and influenza vaccines; other vaccines require a standing order or a prescription in order prior to administration in a pharmacy.
References - Illinois Pharmacy Practice Act (225 ILCS 85) https://ilga.gov/legislation/ilcs/ilcs3.asp?ActID=1318&ChapterID=24
- Illinois Pharmacy Practice Act Administrative Code (Part 1330): https://www.ilga.gov/commission/jcar/admincode/068/06801330sections.html
- Illinois Controlled Substances Act (720 ILCS 570) https://ilga.gov/legislation/ilcs/ilcs5.asp?ActID=1941&ChapterID=53
- Illinois Controlled Substances Act Administrative Code (Part 3100) https://www.ilga.gov/commission/jcar/admincode/077/07703100sections.html
- Pharmacist’s Manual: An Informational Outline of the Controlled Substances Act. Drug Enforcement Administration. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-046R1)(EO-DEA154R1)_Pharmacist%27s_Manual_DEA.pdf
Direct download: 178-mpje.mp3
Category: general
-- posted at: 10:52am EDT
|
|
Tue, 23 January 2024
In this episode, we review evidence-based guidelines for the emergency reversal of warfarin, dabigatran, and the oral Xa inhibitors (apixaban, edoxaban, and rivaroxaban). Key Concepts - Reversal of anticoagulation is indicated in patients with major hemorrhage or when emergency surgery is necessary.
- Reversal of warfarin (Coumadin®) involves a fast-acting, short-term solution (usually prothrombin complex concentrates [PCC]) and a slower-acting, long-term solution (intravenous vitamin K).
- Idarucizumab (Praxbind®) is the preferred reversal strategy for dabigatran (Pradaxa®). Idarucizumab is a monoclonal antibody fragment specific that binds and inactivates dabigatran. If idarucizumab is unavailable, PCCs are recommended.
- Andexanet alfa (Andexxa®) is the preferred reversal strategy for oral Xa inhibitors and has FDA approval specific to apixaban and rivaroxaban. Andexanet alfa is a decoy factor Xa protein with higher binding affinity than human clotting factor Xa. There are several barriers to use with andexanet alfa that has led to low utilization in hospitals. If andexanet alfa is unavailable, PCCs are recommended.
References - Baugh CW, et al. Anticoagulant Reversal Strategies in the Emergency Department Setting: Recommendations of a Multidisciplinary Expert Panel. Ann Emerg Med. 2020;76(4):470-485.
- Cuker A, Burnett A, Triller D, et al. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am J Hematol. 2019;94(6):697-709. doi:10.1002/ajh.25475
- Tomaselli GF, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(5):594-622.
Direct download: 177-anticoag-reversal.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 2 January 2024
In this two part episode, we review some of the most important clinical pearls in the pharmacotherapy and practice aspects of hormonal contraceptives with a brief focus on the very first FDA approved OTC hormonal contraceptive product (Opill). Key Concepts (Part 2) - Missed dose instructions are particularly important with progestin only pills (POPs). Patients should take POPs at the same time (within 3 hours) each day - missing a dose beyond this 3 hour window is considered a missed dose and requires barrier contraception.
- There are a wide variety of hormonal contraception options for patients - each with its own unique advantages and disadvantages. Shared decision making between a healthcare provider and a patient is critical to selecting the most appropriate form of contraception!
- The CDC's Medical Eligibility Criteria (MEC) is an important resource to guide prescribers with regards to selecting hormonal contraception and also in identifying the clinical significance of a variety of drug interactions with hormonal contraception.
- One of the most important aspects of hormonal conctraception is adequate patient follow-up. Especially given the wide variety of hormonal contraception options, patients may need to switch their contraceptive multiple times until they find one that works best for them. Close follow-up and patient counseling are pivotal for helping a patient identify their optimal regimen.
References
Direct download: 176-hormonal-contraception-part-ii.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Wed, 13 December 2023
In this two part episode, we review some of the most important clinical pearls in the pharmacotherapy and practice aspects of hormonal contraceptives with a brief focus on the very first FDA approved OTC hormonal contraceptive product (Opill). Key Concepts (Part 1) - The effectiveness of contraceptives varies based on “ideal use” (e.g. in a clinical trial with optimal compliance) versus “typical use” (e.g. real-world effectiveness in patients who may sometimes be less adherent than in clinical trials). Oral, patch, and ring-based hormonal contraceptives (combination estrogen-progestin or progestin-only formulations) with “typical” use are about ~90% effective, meaning in one year there are ~10 unplanned pregnancies with these contraceptive options.
- When using an estrogen-based oral contraceptive, the estrogen dose should be initiated at a low dose (25 mcg or less per day of ethinyl estradiol). The dose of estrogen may need to be increased if breakthrough bleeding occurs in the early/mid cycle despite being on therapy for at least 6 months.
- Breakthrough bleeding later in the cycle is typically due to an inadequate progestin dose. In general, manufacturers do not provide multiple different formulations with different progestin doses; therefore, if late breakthrough does occur, an alternative formulation with a different progestin should be considered.
- If a patient misses one dose of a combination oral contraceptive, they should take the missed dose as soon as possible (even taking two doses at once if they remember when the next dose is due). If two or more doses are missed, the package insert should be consulted for instructions – management depends on the timing of the cycle, recency of unprotected sex, and other factors.
References
Direct download: 175-hormonal-contraception-part-i.mp3
Category: general
-- posted at: 11:47am EDT
|
|
Mon, 4 December 2023
In this episode, we interview Scott Glosner, PharmD, MPH, BCPS about his extensive experience working at Pfizer in medical outcomes and as a field medical director. Dr. Glosner will share his career journey from a clinical pharmacist transitioning into the pharmaceutical industry in the late 1990s and what current pharmacists and students should know about a job in a pharmaceutical company. Key Concepts - Pharmacists are playing an increasingly important role within the pharmaceutical industry. Prior clinical experience is a significant advantage to applicants for these positions.
- Key characteristics of a competitive pharmacist applicant for an industry position include strong communication skills, being perseverant (“tough skin”), being extremely persistent, and having real-world clinical experience.
- Different companies and job positions within industry often require differing amounts of prior experience. Applicants with more than several years of experience (or equivalent fellowship experience) may be more competitive for positions. Standing out in any way, whether board certification, doing research, networking, etc. is important for any applicant.
- In the future, pharmacists in industry may be playing a greater role in the oncology space, social determinants of health, emerging topics (such as gene therapy), and being capable of analyzing and interpreting “real world” clinical trial data.
Questions for Dr. Scott Glosner? He can be reached at scott.glosner@pfizer.com or on LinkedIn (https://www.linkedin.com/in/scott-glosner-b743234).
Direct download: 174-scott-glosner.mp3
Category: general
-- posted at: 1:22pm EDT
|
|
Tue, 31 October 2023
In this episode, we will discuss the definition of REMS (Risk Evaluation and Mitigation Strategies), why they exist, the role of FDA in administering REMS, types and examples of REMS, and how they impact pharmacy practice. Key Concepts - The REMS (Risk Evaluation and Mitigation Strategies) program was developed in 2007 as part of the FDA’s drug risk management strategies designed to balance risk and benefits of certain drugs.
- Elements of REMS vary depending on the drug, but commonly include medication guides, communication plans, and other elements to assure safe use.
- REMS can require patients, providers, and pharmacies to take certain actions including training, registration, enrollment, safety monitoring, documentation of safety concerns, and follow prescribing and dispensing regulations.
- The FDA captures and assesses data on a regular basis to make changes in the REMS program. It also has authority to enforce compliance and take punitive actions against non-compliant parties.
References
Direct download: 173-REMS.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 10 October 2023
In this episode, we review the role and indications of thrombolytics in acute ischemic stroke. The efficacy, safety, administration considerations, and cost between alteplase and tenecteplase are compared and contrasted. Key Concepts - Alteplase (Activase) is a recombinant DNA version of human TPA (tissue plasminogen activator). Tenecteplase (TNKase) is similar to human TPA except it has three amino acid changes that result in a longer half-life and higher fibrin specificity.
- In patients with stroke, alteplase is given as a bolus followed by a 60-minute infusion. Tenecteplase is given as an IV bolus without the need for an infusion due to its longer half-life.
- Tenecteplase is at least as safe and effective as alteplase in acute ischemic stroke (with some studies showing greater benefit with tenecteplase).
- In patients with acute ischemic stroke who are candidates for mechanical thrombectomy, thrombolytics (with alteplase or tenecteplase) will still be given in patients who meet inclusion criteria and have no exclusion criteria.
References - Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association [published correction appears in Stroke. 2019 Dec;50(12):e440-e441]. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
- Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/NEJMoa1716405
- Kobeissi H, Ghozy S, Turfe B, et al. Tenecteplase vs. alteplase for treatment of acute ischemic stroke: A systematic review and meta-analysis of randomized trials. Front Neurol. 2023;14:1102463. Published 2023 Jan 23. doi:10.3389/fneur.2023.1102463
Direct download: 172-alteplase_vs_tenecteplase.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 19 September 2023
In this episode, we briefly review RSV (respiratory syncytial virus) infections and focus on new data supporting the use of two different RSV vaccines (Abrysvo and Arvexy) in preventing RSV infections in older adults and in pregnant women. Key Concepts - RSV is a contagious respiratory virus that is usually mild and self-limiting in most patients but can cause severe disease especially in young children or older adults with certain risk factors.
- The FDA recently approved two vaccines for RSV (Abrysvo from Pfizer and Arexvy from GSK). The initial FDA approval was for adults 60 years of age and older; however, the FDA recently granted an additional indication for Abrysvo for pregnant women (to prevent the infant from severe RSV infection once born).
- When studied in older adults, both vaccines did meet efficacy criteria but the incidence of RSV infection was relatively low and thus the number needed to treat (NNT) is high. Both studies were done at times with lower RSV prevalence - the NNT would likely be more favorable during RSV outbreaks.
- Unlike Abrysvo, Arvexy (GSK) contains an adjuvant to improve the immune response. Although direct comparisons of efficacy and safety are not appropriate, Arvexy does appear to elicit more systemic adverse effects such as fever, myalgias, headache, and fatigue.
References - Respiratory Syncytial Virus Infection (RSV). Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/index.html
- Abrysvo (respiratory syncytial virus vaccine). US Food & Drug Administration. https://www.fda.gov/vaccines-blood-biologics/abrysvo
- Arexvy (respiratory syncytial virus vaccine, adjuvanted). US Food & Drug Administration. https://www.fda.gov/vaccines-blood-biologics/arexvy
- Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023. Morbidity and Mortality Weekly Report (MMWR). July 21, 2023 / 72(29);793-801. https://www.cdc.gov/mmwr/volumes/72/wr/mm7229a4.htm
- CDC. ACIP Recommendations. Last reviewed August 4, 2023. www.cdc.gov/vaccines/acip/recommendations.html. Accessed August 23, 2023.
- RENOIR - Walsh EE, Pérez Marc G, Zareba AM, et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
- AReSVi-006 - Papi A, Ison MG, Langley JM, et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N Engl J Med. 2023;388(7):595-608. doi:10.1056/NEJMoa2209604
- MATISSE - Kampmann B, Madhi SA, Munjal I, et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023;388(16):1451-1464. doi:10.1056/NEJMoa2216480
- RSV-NET Interactive Dashboard. Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/research/rsv-net/dashboard.html
- ACIP Meeting Information - Meeting Materials. https://www.cdc.gov/vaccines/acip/meetings/index.html
Direct download: 171-rsv.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 29 August 2023
In this episode, together with our faculty colleague, Dr. Roberta Dume, PharmD, BCPP, we discuss the pharmacologic options and evidence for the treatment of opioid use disorder (OUD) and how pharmacists play a vital role in assisting patients suffering from opioid use disorder. Key Concepts - The treatment for OUD should be provided by either the treating clinician or a certified Opioid Treatment Provider (OTP) using one of three FDA-approved therapies which include buprenorphine, methadone, and naltrexone.
- Selection of the OUD treatment depends on availability of treatment provider; pharmacologic agent specific factors such as efficacy, dose titration, safety, and need for detoxification; and patient factors such as ability to safe-keep medications, adherence to required clinic visits, or presence of comorbidities.
- Pharmacists can play an important role for patients needing OUD by providing treatment education, treatment induction, monitoring treatment outcomes, harm reduction by providing naloxone and related education, and utilizing preventative strategies such as monitoring opioid use, offering non-opioid pain management options, and promoting safe storage and disposal of opioids.
References
Direct download: 170-opioid-use-disorder.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 8 August 2023
In this episode, we announce the second iteration of the HelixTalk Drug Superlative Awards -- awards given to medications on the market that are outstanding or notorious. In announcing these completely fictitious awards, we review key clinical pearls and pitfalls that every clinician should be aware of with these notable medications. Key Concepts - The award for the most unique phase III patient population for a widely used medication goes to … Pneumovax-23 (PPSV-23) for its predecessor versions that were studied in South African novice gold miners.
- The award for the most misunderstood boxed warning goes to … all of the DOACs (but specifically apixaban and rivaroxaban). In particular, due to BOTH an increased risk of thrombosis and bleeding when switching from a DOAC to warfarin therapy in patients with atrial fibrillation.
- The award for the biggest difference between pharmacokinetic properties and pharmacodynamic effects goes to … aspirin due to its short-half life and short duration of analgesic effect and yet very prolonged antiplatelet effect.
- The award for the drug that should be dispensed with extra toilet paper … TIE between irinotecan and clindamycin. The most common dose-limiting adverse effect of irinotecan is diarrhea – loperamide is extensively used in these patients. Clindamycin earns the award because it is the antibiotic most associated with Clostridium difficile-associated diarrhea (CDAD).
References - Smit P, Oberholzer D, Hayden-Smith S, Koornhof HJ, Hilleman MR. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA. 1977;238(24):2613-2616.
Farrar JL, Childs L, Ouattara M, et al. Systematic Review and Meta-Analysis of the Efficacy and - Effectiveness of Pneumococcal Vaccines in Adults. Pathogens. 2023;12(5):732. Published 2023 May 19. doi:10.3390/pathogens12050732
- Pavia M, Bianco A, Nobile CG, Marinelli P, Angelillo IF. Efficacy of pneumococcal vaccination in children younger than 24 months: a meta-analysis. Pediatrics. 2009;123(6):e1103-e1110. doi:10.1542/peds.2008-3422
- Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Sferra TJ, Hernandez AV, Donskey CJ. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013 Sep;68(9):1951-61. doi: 10.1093/jac/dkt129.
Direct download: 169-superlatives-2023.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 18 July 2023
There has been a lot of news about abortion (abortifacient) medications recently. Since the overturn of Roe v. Wade in 2022, individual states passed their own laws restricting access to abortion, this includes access to abortion medications. This clearly impacts the way pharmacists practice. In this episode, we summarize the science behind the two main abortive drugs, mifepristone and misoprostol, and provide a picture of how the access to these medications stand in the United States. Key Concepts - Among other modalities to terminate pregnancies, medication abortion is a safe and alternative option that is picking up popularity given recent changes post-Dobbs vs. Jackson WHO decision.
- The FDA-approved use of combination mifepristone and misoprostol regimen to terminate pregnancy up to 70 days (10 weeks of gestation) is based on strong evidence for its efficacy and safety.
- Since the overturning of Roe vs. Wade in 2022, states have taken their own action to further restrict or increase access to abortion services including access to medication abortion.
- These legal changes further impact dispensing of mifepristone and misoprostol by pharmacists across the country adding to more confusion. Legal councils, state boards of pharmacies, or state pharmacy associations may serve as suitable resources to consult regarding these fast-changing laws.
References
Direct download: 168-medication-abortion.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 27 June 2023
In this episode, we review the science behind genetic differences in humans in the CYP2D6 hepatic enzyme responsible for drug metabolism and how these genetic variants can lead to certain drugs being metabolized far too much or far too little, which can cause drug toxicities or a lack of effectiveness. Key Concepts - About 20-25% of drugs on the market are metabolized by CYP2D6. Humans have a huge degree of variability in CYP2D6 metabolism ranging from “ultra” metabolizers to “poor” metabolizers.
- Drugs that heavily rely on CYP2D6 metabolism are prone to large variability in responses due to these genetic differences. Some drugs rely on metabolic inactivation of CYP2D6 whereas other drugs use the enzyme to become converted to a more active compound.
- Codeine and tramadol both heavily rely on CYP2D6 activation to a more potent opioid compound. Patients with excessive CYP2D6 activity will have toxicities (from too much of an active metabolite) whereas patients with low CYP2D6 activity will have little therapeutic effect.
- Numerous antidepressants (paroxetine, nearly all tricyclic antidepressants, and venlafaxine) rely on CYP2D6 metabolism. Differences in CYP2D6 metabolism have been shown to either cause toxicity or a lack of effectiveness with these medications.
References - Chartrand R, Forte AM, Hoger JD, Kane SP, Kisor DF. Pharmacogenomics and Commonly Prescribed Medications. AdvanCE. October 10, 2022. https://www.advancepharmacist.com/courses/pharmacogenomics-and-commonly-prescribed-medications.
- Caudle KE, Sangkuhl K, Whirl-Carrillo M, et al. Standardizing CYP2D6 Genotype to Phenotype Translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin Transl Sci. 2020;13(1):116-124. doi:10.1111/cts.12692
- Bousman CA, Stevenson JM, Ramsey LB, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants [published online ahead of print, 2023 Apr 9]. Clin Pharmacol Ther. 2023;10.1002/cpt.2903. doi:10.1002/cpt.2903
- Crews KR, Monte AA, Huddart R, et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin Pharmacol Ther. 2021;110(4):888-896. doi:10.1002/cpt.2149
Direct download: 167-pgx-of-2d6.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 6 June 2023
In this episode, we compare hydrochlorothiazide and chlorthalidone, but specifically from a cardiovascular outcomes perspective when used in patients with hypertension. Key Concepts - Chlorthalidone, hydrochlorothiazide, and indapamide are available thiazide diuretics for treatment of hypertension; however, hydrochlorothiazide is the most commonly used agent.
- Chlorthalidone is more potent in reducing blood pressure but also is associated with a higher risk of electrolyte abnormalities compared to HCTZ.
- Recent studies for cardiovascular outcomes show that chlorthalidone is not better than HCTZ in preventing CV outcomes, but increases risk for hypokalemia, need for monitoring and even potassium supplementation.
References
Direct download: 166-thiazide-throwdown.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 16 May 2023
In this episode, we discuss the concerns of QTc prolongation, which can cause a fatal arrhythmia called torsades de pointes (TdP). We cover the difference between QT and QTc, how to interpret a QTc (and when it is inaccurate), common medications that prolong QTc, and how pharmacists can evaluate the risk of QTc/TdP in patients who are receiving QTc-prolonging therapies. Key Concepts - The QTc interval is the QT interval that has been “corrected” for heart rate. In nearly all cases, when describing a QT interval, it should be expressed as the QTc.
- Although a prolonged QTc is usually defined as a QTc exceeding 450-480 msec, the risk of torsades de pointes (TdP) begins to become concerning when the QTc is more than 500 msec, 15-20% longer than baseline, or if the QTc has increased by more than 60 msec.
- Vaughan-Williams Class III antiarrhythmics are most implicated in QTc prolongation and TdP risk. These therapies include sotalol, dofetilide, and dronedarone. Although amiodarone is a class III antiarrhythmic, its risk of TdP is quite low despite the fact that it often substantially prolongs the QTc.
- When pharmacists are assessing the risk of QTc prolongation and TdP, multiple factors (not just the QTc itself) should be considered. Risk scores, like the Tisdale Risk Score, as well as considering the risks/benefits of switching drug therapy, should be evaluated.
References
Direct download: 165-qtc.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 25 April 2023
In this episode, we will discuss the most important updates from the American Diabetes Association’s 2023 Standards of Care in Diabetes. Key Concepts - The first-line therapy for type II diabetes is based on whether the primary goal of therapy is cardiorenal benefit (reduced risk of ASCVD, heart failure, or CKD) or glycemic and weight goals.
- For cardiorenal benefit, GLP1 receptor agonists and SGLT2 inhibitors are heavily emphasized. For glycemic control and weight gain, GLP1 receptor agonists (or GLP1/GIP in the case of tirzepatide) have a very favorable effect on weight loss and glycemic control. While metformin is still mentioned, it is no longer the sole, first-line therapy for type II diabetes.
- For patients with diabetes and a high risk of ASCVD (20% or higher), high-intensity statins, ezetimibe, and/or PCSK9 inhibitors are recommended to achieve an LDL less than 70 mg/dL. In patients with a history of ASCVD events, these same therapies are used to achieve a recommended LDL goal of less than 55 mg/dL.
- Among selected patients with diabetes and CKD with albuminuria, finerenone (a new mineralocorticoid receptor antagonist) is recommended to improve renal and cardiovascular outcomes.
- A variety of different therapies are now recommended for neuropathic pain, including gabapentinoids, SNRIs, TCAs, and several antiseizure medications (lamotrigine, lacosamide, oxcarbazepine, and valproic acid).
- A wide variety of other new recommendations are discussed in the episode, including NASH/NAFLD, obesity and weight management, special populations, diabetes technology, and health behavior changes.
References
Direct download: 164-diabetes-2023.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 4 April 2023
In this episode, we review clinical pearls and common pitfalls of immunosuppression regimens for organ transplantation with a particular focus on tacrolimus and mycophenolate. Key Concepts - Most recipients of an organ transplantation will be on a two or three drug regimen. The most common regimen is tacrolimus and mycophenolate with/without a corticosteroid.
- Tacrolimus is hepatically eliminated and susceptible to CYP3A4 and PGP drug interactions. Particularly at higher drug concentrations, it is associated with nephrotoxicity and neurotoxicity (among several other adverse effects).
- Mycophenolate is unstable in the acidic environment of the stomach. The two formulations on the market are CellCept (which uses a prodrug, mycophenolate mofetil, that is converted in the liver to an active compound) and Myfortic (an enteric-coated formulation of mycophenolic acid, which releases after exiting the stomach).
- The intensity of an immunosuppression regimen is determined by numerous factors, including the type of organ, how long ago the organ was transplanted, if acute rejection has occurred in the past, patient-specific risk factors, and more.
Additional Resources - Register to be a donor at Donate Life America (https://donatelife.net) or at the HRSA OrganDonor.gov site (https://www.organdonor.gov)
- Learn more about stem cell donation and transplant at https://bethematch.org
Direct download: 163-transplant-tango.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 14 March 2023
In this first ever CE episode, we discuss the A-Zs of continuous glucose monitors (CGMs). In specific, our learning objective for the CE are: -
Describe commonly available types of continuous glucose monitors (CGMs) in the US market and the features and capabilities of these devices. -
Summarize the evidence and guideline recommendations for use of CGMs in the management of diabetes. -
Identify the role of the pharmacist in the selection of CGMs and provision of education to patients and providers. -
Interpret the ambulatory glucose profile (CGM data output) and recommend changes in antihyperglycemic regimen for a patient. ACPE-Accredited Pharmacist CE (1.0 hrs) To obtain CE credit for a $5 fee, visit the following link: https://rfums.wufoo.com/forms/z1qzh5vf0ggr832/. Once payment is successful, you will be redirected to our CE partner (CE Impact) to complete an evaluation and to earn 1.0 hour of CE credit. CE is available for 12 months after episode publication. Key Concepts - There are two main types of stand-alone personal CGMs available in the US market – real-time (rtCGM) and intermittently scanning (isCGM). [1] These CGMs vary in their features such as sensor wear time, sensor warm up time, sensor application site, reader availability, approved age for use, fingerstick calibration, non-adjunctive FDA labeling, interconnectability with other technology such as insulin pumps, and drug interactions – these variabilities can be used in decision-making when selecting an appropriate CGM for a patient. [2-7]
- Based on the evidence for use, both types of CGMs (real-time and intermittently scanning) are recommended in patients with Type 1 and Type 2 diabetes who are on multiple-daily insulin or continuous insulin infusion (pump), patients with Type 2 diabetes on basal insulin therapy, and as adjunct use in patients with diabetes who are pregnant. The strength of recommendations in general is stronger for real-time CGMs than for intermittently scanning CGMs. [1,11] These recommendations are supported by the evidence that CGMs can help improve glucose control, reduce risk of hypoglycemia, diabetes-related hospitalizations, and patient/caregiver satisfaction.
- Pharmacists play an integral role in education, on-going support, data interpretation, and resulting disease management in patients who qualify for CGM use and providers who care for patients with diabetes. [14]
- The ambulatory glucose profile is a standardized data output that informs understanding of glucose trends. [15] The recommended goal for most patients is to maintain a glucose range between 70-180 mg/dL with at least 70% of time spent in this range with variability coefficient of no more than 36%. [1,11,15]
Supplemental Content Comparison of rtCGM and isCGM devices 
"Mary's" Example AGP Report (adapted from Battelino et al.) 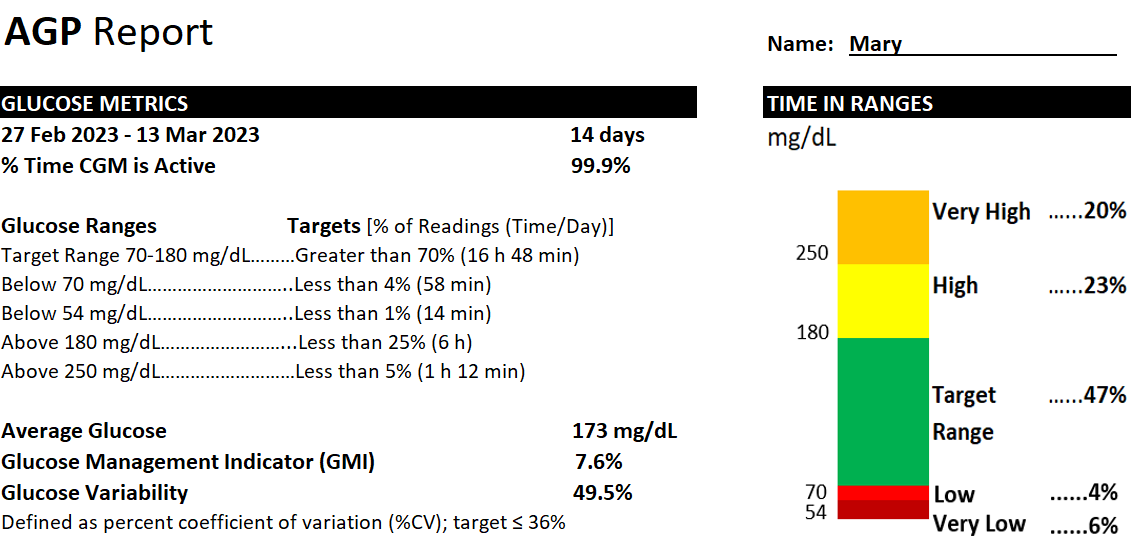 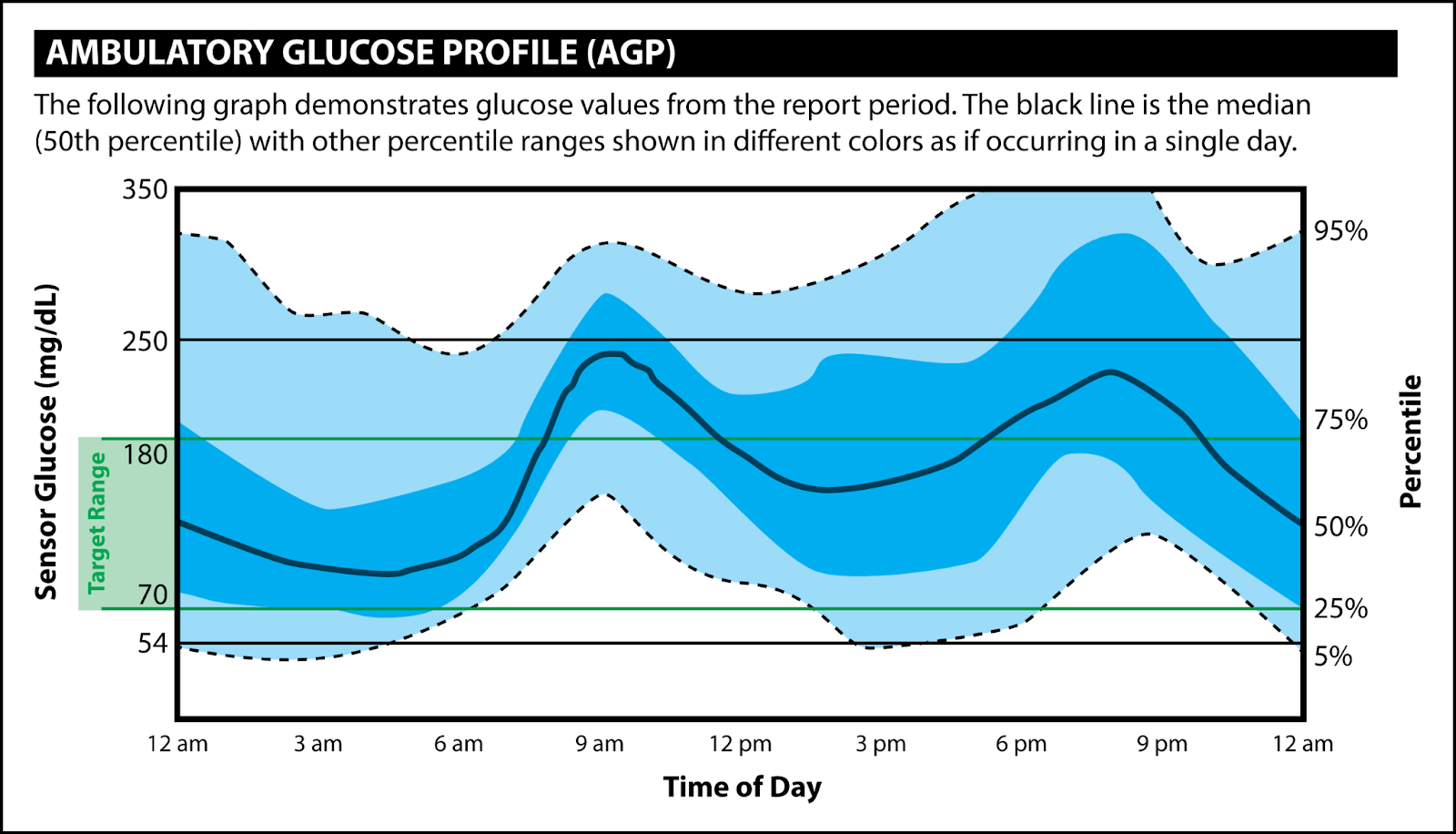
References - ElSayed NA, Aleppo G, Aroda VR, et al. American Diabetes Association. Chapter 7. Diabetes technology: Standards of medical care in diabetes - 2023. Diabetes Care. 2023;46(suppl 1):S111-S127.
- Dexcom G6 User Guide. Dexcom, Inc. 2020. Accessed February 20, 2023. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf.
- Dexcom G7 User Guide. Dexcom, Inc. 2022. Accessed February 20, 2023. https://dexcompdf.s3.us-west-2.amazonaws.com/en-us/G7-CGM-Users-Guide.pdf#page=12
- Guardian Connect System User Guide. Medtronic MiniMed. 2020. Accessed February 20, 2023. https://www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf.
- Eversense E3 User Guide. Sensionics, Inc. 2022. Accessed February 20, 2023. https://www.eversensediabetes.com/wp-content/uploads/LBL-4002-01-001-Rev-F_Eversense-E3-User-Guide_mgdL_R1_web.pdf
- FreeStyle Libre 3 User’s Manual. Abbott Diabetes Care Inc. 2022. Accessed February 20, 2023. https://freestyleserver.com/Payloads/IFU/2022/q2/ART44140-002_rev-A.pdf
- FreeStyle Libre 2 User’s Manual. Abbott Diabetes Care Inc. 2020. Accessed February 20, 2023. https://freestyleserver.com/Payloads/IFU/2020/q2/ART40703-001_rev-D-Web.pdf.
- Products. American Diabetes Association. Accessed February 20, 2023. https://consumerguide.diabetes.org/
- Wood A, O'Neal D, Furler J, Ekinci EI. Continuous glucose monitoring: a review of the evidence, opportunities for future use and ongoing challenges. Intern Med J. 2018 May;48(5):499-508.
- Edelman SV, Argento NB, Petty SJ, Hirsch IB. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care. 2018;41:2265-2274.
- Fonseca VA, Grunberger G, Anhalt H, et al. Continuous glucose monitoring: A consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22(8):1008-21.
- Reiterer F, Polterauer P, Schoemaker M, Schmelzeisen-Redecker G, Freckmann G, Heinemann L, Del Re L. Significance and Reliability of MARD for the Accuracy of CGM Systems. J Diabetes Sci Technol. 2017 Jan;11(1):59-67. doi: 10.1177/1932296816662047. Epub 2016 Sep 25. PMID: 27566735; PMCID: PMC5375072.
- Food and Drug Administration. Premarket Notification 510(k). 2022. Accessed February 25, 2023. https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k.
- Isaacs, Diana. The pharmacist’s role in continuous glucose monitoring. Pharmacy Today. 2020;26:37-54.
- Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603.
Direct download: 163-cgm.mp3
Category: general
-- posted at: 7:00am EDT
|
|
Tue, 21 February 2023
In this episode, we interview Dr. Shannon Rotolo and Dr. Alex Berce regarding Illinois and Wisconsin drug repository programs – these are programs that allow certain medications to be donated to participating sites and then redistributed to patients at a very low dispensing cost. Key Concepts - Drug repository programs allow participating sites to accept certain donated medications and redistribute these medications to needy patients at a very low dispensing cost.
- Drug repository programs are regulated by state law and the specifics of the process do vary by state. In Illinois and Wisconsin, donated medications must be in their original containers with tamper-evident packaging, cannot be controlled substances, and must have a 90-day expiration window at the time of donation.
- Pharmacists can play an important role in advocating for patients and the profession of pharmacy. The involvement of pharmacists in legislation is critical to make sure that new laws are actually “functional” and can achieve their intended purpose.
References For additional information about our guests, contact Dr. Shannon Rotolo at Shannon.Rotolo@uchospitals.edu or Dr. Alex Berce at alex@goodvaluerx.com.
Direct download: 161-drug-repositories.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 31 January 2023
In this episode, we discuss the evidence, safety, and place in therapy of Auvelity® (dextromethorphan-bupropion), a newly approved antidepressant with a unique mechanism of action and interesting pharmacokinetic considerations. Key Concepts - Auvelity® (bupropion-dextromethorphan) was FDA approved in 2022 for major depressive disorder (MDD). The bupropion component inhibits CYP2D6 metabolism and increases serum concentrations of dextromethorphan. The proposed mechanism of benefit in MDD is via dextromethorphan (as an NMDA antagonist) and possibly with bupropion (as a dopamine/norepinephrine reuptake inhibitor).
- Although the bupropion component in Auvelity® is being used for its drug interaction, the dose is a therapeutic dose and carries several warnings and precautions, including the risk of seizure and hypertension.
- In short (6-week) clinical trials, Auvelity® improved depression symptoms quickly (within 1-2 weeks), which is faster than many other antidepressants. Auvelity® is associated with dizziness, anxiety, hyperhidrosis, nausea, headache, diarrhea, and dry mouth.
- As a CYP2D6 inhibitor, the bupropion component of Auvelity® will cause drug interactions with many other medications, including some antidepressants, antipsychotics, and opioid analgesics (among others).
References - Iosifescu DV, Jones A, O'Gorman C, et al. Efficacy and Safety of AXS-05 (Dextromethorphan-Bupropion) in Patients With Major Depressive Disorder: A Phase 3 Randomized Clinical Trial (GEMINI). J Clin Psychiatry. 2022;83(4):21m14345. Published 2022 May 30. doi:10.4088/JCP.21m14345
- Tabuteau H, Jones A, Anderson A, Jacobson M, Iosifescu DV. Effect of AXS-05 (Dextromethorphan-Bupropion) in Major Depressive Disorder: A Randomized Double-Blind Controlled Trial. Am J Psychiatry. 2022;179(7):490-499. doi:10.1176/appi.ajp.21080800
- Kotlyar M, Brauer LH, Tracy TS, et al. Inhibition of CYP2D6 activity by bupropion. J Clin Psychopharmacol. 2005;25(3):226-229. doi:10.1097/01.jcp.0000162805.46453.e3
Direct download: 160-auvelity.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 10 January 2023
In this episode, we highlight important changes to the 2023 GOLD Guidelines for COPD. In particular, we discuss a revision to the GOLD group classification system and the preferred initial therapies in patients with COPD. Key Concepts - The newest GOLD COPD guidelines now recognize three GOLD groups – “A”, “B”, and “E”. Group “E” (formerly groups C and D) are patients with frequent exacerbations (defined as 2 or more in the past 12 months or 1 exacerbation requiring hospitalization).
- For group “E” patients, the preferred initial inhaler regimen is a LABA+LAMA. Triple therapy (LABA+LAMA+ICS) can be considered if blood eosinophils are elevated.
- “Triple therapy” (LABA+LAMA+ICS) has gained traction based on the IMPACT and ETHOS trials – this regimen reduced exacerbations and mortality compared to LABA+LAMA and LABA+ICS.
- With an exploding market of new COPD inhalers, the role of the pharmacist is even more critical to help identify affordable medications and provide patient education for proper inhaler technique.
References - Global Strategy for Prevention, Diagnosis, and Management of COPD: 2023 Report. Global Initiative for Chronic Obstructive Lung Disease (GOLD). https://goldcopd.org/2023-gold-report-2/
- IMPACT study: Lipson DA, Barnhart F, Brealey N, et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N Engl J Med. 2018;378(18):1671-1680. doi:10.1056/NEJMoa1713901
- ETHOS study: Rabe KF, Martinez FJ, Ferguson GT, et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N Engl J Med. 2020;383(1):35-48. doi:10.1056/NEJMoa1916046
Direct download: 159-gold-2023.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Wed, 21 December 2022
In this episode, we will discuss all things peripheral arterial disease – definitions, staging, clinical presentation, risk factors, goals of therapy, and guideline-directed medication therapy recommendations including the newest evidence for the use of DOACs. Key Concepts - Addressing modifiable risk factors (weight loss, smoking cessation, blood pressure and blood glucose control, dyslipidemia, structured exercise program, etc.) are recommended for the treatment of PAD.
- Single antiplatelet therapy with either aspirin 81 mg or clopidogrel 75 mg daily are recommended in patients to reduce stroke, MI and other vascular deaths in symptomatic (1A) and asymptomatic patients (IIa- C-EO).
- Rivaroxaban 2.5 mg BID, when added to aspirin 81 mg daily, is superior to aspirin alone in preventing composite outcome of stroke, MI, and CV death in PAD patients with recent revascularization surgery for PAD but increases the risk of major bleeding.
- In the absence of heart failure, cilostazol is effective in improving symptoms, quality of life, and increasing walking distance in patients with intermittent claudication.
References - Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. https://doi.org/10.1161/CIR.0000000000000470
- Criqui MH, Matsushita K, Aboyans V, et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation. 2021;144:e171–e191. https://doi.org/10.1161/CIR.0000000000001005
- Alonso-Coello P, Bellmunt S, McGorrian C, Anand SS, Guzman R, Criqui MH, Akl EA, Vandvik PO, Lansberg MG, Guyatt GH, Spencer FA. Antithrombotic therapy in peripheral artery disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e669S-e690S. doi: 10.1378/chest.11-2307. PMID: 22315275; PMCID: PMC3278062.
- Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017; 377:1319-1330. https://www.nejm.org/doi/full/10.1056/nejmoa1709118
- Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral arterial disease after revascularization. N Engl J Med. 2020; 382:1994-2004. https://www.nejm.org/doi/full/10.1056/nejmoa2000052
Direct download: 158-pad.mp3
Category: general
-- posted at: 7:00am EDT
|
|
Tue, 29 November 2022
In this episode, we review the management of a patient with hypokalemia, including both inpatient and outpatient supplementation with potassium chloride supplements and what dosage forms are available for potassium repletion. Key Concepts - Most diets will provide sufficient potassium to avoid hypokalemia. Hypokalemia usually occurs due to drug therapy (such as diuretics) or GI losses from severe vomiting or diarrhea.
- In patients with chronically low potassium, supplements are dosed to increase dietary intake of potassium by about 20-40 mEq per day. For acute repletion, 10 mEq of potassium should increase serum potassium by about 0.1 mEq/L.
- Over-the-counter potassium (as potassium gluconate) contains a very small amount of potassium (2.5 mEq). Potassium chloride powders and liquids (like salt substitutes) taste terrible and are poorly tolerated. Most patients will replete potassium via slow-release tablets (Klor-Con or Klor-Con M) or via potassium chloride IV infusions.
- Most IV fluids do not contain any potassium at all (or very little potassium). Patients receiving these IV fluids who are NPO will eventually become hypokalemic. Certain maintenance fluids do contain potassium – most patients will receive about 40 mEq of potassium per day with these IV fluids.
Direct download: 157-potassium.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 8 November 2022
In this episode, we will define Digital Health, its categories and examples, describe how pharmacists are involved in DH practice, opportunities and limitations and future of DH. We will also discuss what implications DH has for educators, educational institutions, student pharmacists, pharmacists, and practice of pharmacy in general. Key Concepts - Digital Health is currently a broad umbrella category that uses mobile health, telehealth, web-based platforms, personalized medicine, and IT to provide scalable patient care.
- There are several focused areas within DH that would impact pharmacy practice by warranting pharmacist oversight or collaborative insights.
- There is positive data for pharmacist-led DH interventions using mobile apps and web-based tools, but the use of telehealth modality has mixed results.
- Pharmacists need to stay current in their knowledge and skills for utilizing DH tools in integrative and collaborative patient care.
References - Aungst TD, Franzese C, Kim Y. Digital health implications for clinical pharmacists services: A primer on the current landscape and future concerns. J Am Coll Clin Pharm. 2020;4(4):514-524. DOI: 10.1002/jac5.1382. https://accpjournals.onlinelibrary.wiley.com/doi/abs/10.1002/jac5.1382
- American Association of Colleges of pharmacies. Digital Health Workshop - Resources. https://www.aacp.org/resource/digital-health-workshop-resources (Lists resources from Digital Therapeutics Alliance and Digital Medicine Society)
- Park T, Muzumdar J, Kim H. Digital Health Interventions by Clinical Pharmacists: A Systematic Review. Int J Environ Res Public Health. 2022 Jan 4;19(1):532. doi: 10.3390/ijerph19010532. PMID: 35010791; PMCID: PMC8744767.
Direct download: 156-digital-health.mp3
Category: general
-- posted at: 5:00am EDT
|
|
Tue, 18 October 2022
In this episode, we invite Dr. Amir Ali, PharmD, BCOP to discuss with us the pathophysiology, risk factors, prevention, and treatment clinical pearls of tumor lysis syndrome TLS). Key Concepts - TLS is caused by rapid cell death of cancerous cells that results in intracellular contents “spilling” into the blood – this leads to high serum uric acid, high serum potassium, high serum phosphate, and LOW calcium.
- These laboratory abnormalities cause acute kidney injury (via crystal formation in the kidney), arrhythmias (from hyperkalemia), and seizures (from high phosphate and low calcium).
- Patients at highest risk for TLS are those with hematologic malignancies (lymphomas and leukemias), especially if WBC or LDH labs are very high.
- Prevention is the Key! The primary prevention approach for TLS is hydration, allopurinol, and sometimes a low dose of rasburicase. The treatment of TLS involves more aggressive hydration and rasburicase.
References - Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review [published correction appears in J Clin Oncol. 2010 Feb 1;28(4):708]. J Clin Oncol. 2008;26(16):2767-2778. doi:10.1200/JCO.2007.15.0177
- Cairo MS, Coiffier B, Reiter A, Younes A; TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149(4):578-586. doi:10.1111/j.1365-2141.2010.08143.x
- Jones GL, Will A, Jackson GH, Webb NJ, Rule S; British Committee for Standards in Haematology. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169(5):661-671. doi:10.1111/bjh.13403
Direct download: 155-tls.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 27 September 2022
In this episode, we review Paxlovid (nirmatrelvir/ritonavir) from the perspective of its pharmacology, efficacy, safety, pharmacists’ authority to prescribe, drug interactions, and rebound symptoms after Paxlovid therapy. Key Concepts - Paxlovid is the preferred outpatient therapy for COVID-19 in patients at high risk for progressing to severe COVID-19. It likely has similar efficacy to IV monoclonal antibodies and IV outpatient remdesivir but differences in vaccination rates and patient populations makes a direct comparison difficult.
- The 5-day course of Paxlovid is generally well tolerated. “Paxlovid mouth” (dysgeusia) is relatively common and is characterized by a terrible metallic or garbage-like taste in the mouth during therapy.
- As of July 2022, licensed pharmacists have the authority to assess patients for Paxlovid and prescribe the therapy; however, Medicare/Medicaid reimbursement has not clearly established how reimbursement of clinical services can occur.
- “Rebound” COVID-19 symptoms may or may not be due to Paxlovid (versus the natural course of the disease). If rebound symptoms occur, they are almost always mild or asymptomatic in nature and do not require additional treatment.
References
Direct download: 154-paxlovid.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 6 September 2022
In this episode, we will discuss mechanism, pharmacokinetics, efficacy, safety, and possible place in therapy for tirzepatide (Mounjaro), a new treatment for type 2 diabetes. Key Concepts - Tirzepatide is a novel GIP and GLP-1 receptor agonist resulting in glucose-dependent secretion of insulin and a decrease in glucagon secretion.
- This medication was FDA approved in May 2022 for the treatment of type 2 diabetes as an adjunct to diet and exercise. It is available as a long-acting once weekly pen injection to be administered subcutaneously.
- Current efficacy data exist from a 40-week trial which showed that tirzepatide was superior to semaglutide in A1c reduction and weight loss.
- The most common adverse effects of tirzepatide include GI concerns such as nausea, vomiting, and diarrhea as well as hypoglycemia.
References - Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385:503-515. https://www.nejm.org/doi/full/10.1056/NEJMoa2107519
- Mounjaro. Package insert. Elli Lilly and Company. 2022.
Direct download: 153-tirzepatide.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 16 August 2022
In this episode, we will discuss the rationale behind the FDA approval of two new pneumococcal conjugate vaccines (PCV20 and PCV15), the characteristics of these vaccines, their place in therapy as recommended by the ACIP, and subsequent CDC immunization schedule changes. Key Concepts - Pneumococcal disease is mainly caused by various serotypes of Streptococcus pneumoniae and presentation can vary from mild forms (sinusitis, otitis media) to more severe (pneumonia, bacteremia, or meningitis).
- Previously we used PCV13 and PPSV23 vaccines for adults ages 18 years and older for prevention of pneumococcal disease, but the recommendations were rather complicated based on age, underlying condition/immune status, and vaccination status.
- Two new conjugate-type pneumococcal vaccines, PCV20 (Prevnar 20) and PCV15 (Vaxneuvance) are now approved by the FDA and were recently added to the CDC’s adult immunization schedules.
These updated recommendations are more simplified where adults with high-risk conditions and those ages 65 years and older should receive either 1 dose of PCV20 vaccine or 1 dose of PCV15 and then 1 dose of PPSV23 a year later to complete their pneumococcal vaccine series. - PCV15 is now FDA approved for children and updated recommendations for children have been voted upon by the Advisory Committee on Immunization Practices (ACIP) and will be final once it is made official policy by the CDC.
References and Resources - Kobayashi M, Farrar JL, Gierke R, Britton A, Childs L, Leidner AJ, et al. Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR. 2022;71(4);109–117. https://www.cdc.gov/mmwr/volumes/71/wr/mm7104a1.htm?s_cid=mm7104a1_w
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 14th ed. Hall E., Wodi A.P., Hamborsky J., et al., eds. Washington DC: Public Health Foundation; 2021.
- Goldblatt D, O’Brien KL. Pneumococcal Infections. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson J. eds. Harrison's Principles of Internal Medicine 21e. McGraw Hill; 2022. Accessed August 04, 2022.
- Wagner AL, Boulton ML. Pneumococcal Infections. In: Boulton ML, Wallace RB. eds. Maxcy-Rosenau-Last Public Health & Preventive Medicine, 16e. McGraw Hill; 2022. Accessed August 04, 2022.
- CDC’s PneumoRecs VaxAdvisor mobile app: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/pneumoapp.html
- CDC’s Pneumococcal vaccine timing for adults: https://www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf
Direct download: 152-new-pneumococcal-vaccines.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 26 July 2022
In this episode, we “deep dive” into diltiazem, describing its most important drug facts, pharmacology and medicinal chemistry, pharmaceutics, AB compatibility, and important medication safety issues. Key Concepts - Diltiazem is a non-dihydropyridine calcium channel blocker (CCB). This type of CCB reduces both heart rate and blood pressure whereas dihydropyridine CCBs only reduce blood pressure.
- Diltiazem has numerous dosage forms (IV, immediate release tablets, and extended-release products). Extended-release products are always dosed once or twice daily. Historically there were a significant number of extended-release capsules with a variety of brand names and AB-compatibility. Today, only a few branded products still exist in the US market (Cardizem CD, Cartia XT, Cardizem LA, Tiazac, Taztia XT).
- The FDA Orange Book describes “AB” compatibility, which outlines whether one formulation is therapeutically equivalent to another formulation. Depending on state law, pharmacists can use AB compatibility codes to automatically substitute formulations without notifying the prescriber.
- The numerous dosage forms of diltiazem is a medication safety issue. Remember that immediate release diltiazem is always dosed TID/QID (3-4 times per day) whereas extended-release formulations are always dosed once daily. A twice-daily extended-release product was previously on the market but has since been discontinued.
Direct download: 151-diltiazem.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 5 July 2022
Direct download: 150-reflections.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 14 June 2022
In this episode, we will cover a complete overview of definition, diagnosis, treatment and monitoring of iron deficiency anemia (IDA). Key Concepts - Iron-deficiency anemia (IDA) is the most common type of nutritional anemia. The most common risk factors are insufficient dietary intake, malabsorption, and increased requirement states like pregnancy or blood loss.
- Serum ferritin serves as the most confirmatory lab test for diagnosis of IDA. A low serum ferritin level usually indicates the presence of IDA. Other iron studies and CBC can be helpful in diagnosing IDA as well.
- Generally oral iron therapy is a well-accessible, inexpensive, safe, and effective approach for IDA treatment. Almost all PO options are equally effective and safe. Gastrointestinal adverse effects are common and can sometimes limit further dosing.
- Intravenous iron therapy is generally reserved for patients who are refractory or intolerant to PO treatment, have malabsorption of PO iron therapy, or have other health conditions such as chronic kidney disease, cancer, upcoming surgery, etc. Available IV options are equally effective and selection of an agent depends on insurance coverage, formulary inclusion, patient preference for test dose, frequency of dosing, etc.
Direct download: 149_-_iron_deficiency_anemia.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 24 May 2022
In this episode, we interview Hetal Patel, PharmD and RFUMS COP Alumni, regarding her career path that eventually led her to open Lebanon Family Pharmacy in TN in 2021. We discuss the challenges and opportunities of starting a new independent pharmacy and what the future of independent pharmacy looks like. Key Concepts - Starting a new, independent pharmacy requires substantial planning 8 to 12 months before the pharmacy’s doors even open. New pharmacy owners need to consider a variety of factors such as location, type of building, a business plan with financial analysis, a variety of building and pharmacy inspections, paperwork and government approvals, and so much more.
- PSAOs (pharmacy services administrative organizations) can be helpful, especially for new pharmacy owners, to serve as a liaison between the pharmacy and PBMs (pharmacy benefit managers) to negotiate reimbursement contracts. As owners gain more experience, there may be financial advantages to not using PSAOs and negotiating with PBMs directly.
- Companies like “Health Mart” have a franchise-like model to provide products, services, documentation, policies and procedures, and more to independent pharmacies. These companies reduce the workload associated with running a pharmacy so that the pharmacy owners can focus their time and attention on the business itself and providing exceptional customer service.
- There are a number of challenges to independent pharmacies – some of these challenges involve PBMs (DIR fees and MAC pricing) as well as unrestricted dispensaries in primary care clinics.
References
Direct download: 148_-_hetal_patel.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 3 May 2022
In this episode, we review new updates and key concepts from the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. This guideline is newly published (April 2022) and is a full update of the 2013 guidelines and the 2017 focused update for heart failure. Key Concepts - Heart failure is classified as HFrEF (heart failure with reduced ejection fraction <= 40%), HFimpEF (with improved ejection fraction – was <=40% but is now > 40%), HFmrEF (ejection fraction 41% to 49% with increased LV filling pressures), and HFpEF (preserved ejection fraction >= 50% with increased LV filling pressures). Most drug therapy recommendations are similar for HFrEF, HFimpEF, and HFmrEF whereas HFpEF therapies are different.
- The 2022 AHA/ACC/HFSA heart failure guidelines now recommend SGLT2 inhibitors, such as dapagliflozin and empagliflozin, in patients with HFrEF, HFmrEF, and HFpEF.
- The 2022 AHA/ACC/HFSA heart failure guidelines continue to prefer ARNi, such as sacubitril/valsartan (Entresto), over ACE inhibitors and ARBs in patients with HFrEF. Based on the PARAGON-HF trial, ARNi is also recommended in those with HFpEF albeit with a weak recommendation.
- Avoiding excessive dietary sodium is reasonable to reduce congestive symptoms in patients with heart failure; however, guidelines do not recommend a specific maximum intake nor does data support clinical outcome benefit with dietary sodium restriction.
References - Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [published online ahead of print, 2022 Apr 1]. Circulation. 2022;101161CIR0000000000001063. doi:10.1161/CIR.0000000000001063. https://www.ahajournals.org/doi/10.1161/CIR.0000000000001063
- Ezekowitz JA, Colin-Ramirez E, Ross H, et al. Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): an international, open-label, randomised, controlled trial. Lancet. 2022;399(10333):1391-1400. doi:10.1016/S0140-6736(22)00369-5
Direct download: 147_-_2022_hf_guidelines.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 12 April 2022
In this episode, we discuss six newer antibiotics that target multidrug resistant gram negative bacteria with Dr. Christie Bertram, PharmD, BCIDP. We review common resistance mechanisms, particularly to carbapenems, and highlight the current role in therapy for the following antibiotics: ceftolozane/tazobactam (Zerbaxa®), ceftazidime/avibactam (Avycaz®), meropenem/vaborbactam (Vabomere®), imipenem/cilastatin/relebactam (Recarbrio®), cefiderocol (Fetroja®), and eravacycline (Xerava®). Key Concepts - Ceftolozane/tazobactam (Zerbaxa®) is primarily used for multidrug resistant Pseudomonas; it does not cover carbapenemase-producing organisms and (despite the tazobactam) needs metronidazole for intra-abdominal anaerobic coverage.
- Ceftazidime/avibactam (Avycaz®) is primarily used to cover CRE (Carbapenem-resistant Enterobacterales) but also has activity for many other gram negatives except Acinetobacter.
- Meropenem/vaborbactam (Vabomere®) has similar coverage to Avycaz® but may provide coverage for certain KPCs (Klebsiella pneumoniae carbapenemase). Vaborbactam does not restore activity for meropenem-resistant Pseudomonas.
- Imipenem/cilastatin/relebactam (Recarbrio®) has similar coverage to Avycaz® and Vabomere®; true niche in therapy is not yet well defined.
- Cefiderocol (Fetroja®) uses a unique mechanism to enter gram negative bacteria and has a broad spectrum of activity against carbapenemase-producing bacteria and many other multidrug resistant gram negatives. It has no gram positive activity.
- Eravacycline (Xerava®) is a tigecycline-like tetracycline with a broad spectrum of activity against carbapenemase-producing gram negative, gram positive, an anaerobic bacteria EXCEPT it lacks coverage for Pseudomonas.
References - Yusuf E, Bax HI, Verkaik NJ, van Westreenen M. An Update on Eight "New" Antibiotics against Multidrug-Resistant Gram-Negative Bacteria. J Clin Med. 2021;10(5):1068. Published 2021 Mar 4. doi:10.3390/jcm10051068
- CDC Antibiotic Resistance Threats in the United States, 2019 report. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
Direct download: 146_-_new_antibiotics.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 22 March 2022
In this episode, we bring in two guests to discuss the impact of professional advocacy and resulting professional advancements in the state of Illinois. These guests were the front-line agents of advocacy which resulted in pharmacists' ability to prescribe hormonal contraceptives for patients in Illinois (HB 135). We take a deep dive into their efforts to make this change possible, how it will impact patient care, and its implications on possibilities for further advancement of the pharmacy profession all the while highlighting the importance of professional advocacy.
Direct download: 145_-_pharmacist_oral_contraception.mp3
Category: general
-- posted at: 7:00am EDT
|
|
Tue, 1 March 2022
In this episode, we interview Dr. Danyelle Martin, a Medical Science Liaison (MSL) at Moderna, in order to learn more about the what, how, and future of mRNA-based therapeutics, and what impact it can have on healthcare and healthcare professionals in general. Key Concepts - Moderna has a “Research Engine” proprietary service that takes an mRNA idea from a web-based digital designer, to a digital ordering system, and finally to a production facility where mRNA constructs are synthesized and quality tested.
- Pharmaceutics play a big role in the formulation of mRNA particles. Lipid nanoparticles (LNPs) play an important role for stability and delivery of mRNA cargo. After LNPs and mRNA are co-formulated, the product is purified, filtered, frozen, and subjected to a series of good manufacturing practice (GMP) tests to ensure product quality.
- COVID-19 vaccines are a small glimpse into the potential future of mRNA-based therapeutics. Moderna’s pipeline includes mRNA vaccines for other viruses (including RSV, influenza, Zika and CMV) as well as therapeutics for non-viral diseases (including a personalized cancer vaccine and a VEGF-A mRNA molecule for myocardial ischemia).
Direct download: 144_-_moderna_mrna.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 8 February 2022
In this episode, we review the diagnostic criteria and treatment strategy of hypothyroidism including the controversy surrounding brand versus generic levothyroxine and non-levothyroxine thyroid drugs. Key Concepts - The most common cause of hypothyroidism is autoimmune thyroiditis - the body attacks the thyroid gland cells. Typically in hypothyroidism, TSH levels will be high and thyroid hormone levels (T3 and T4) will be normal or low.
- Levothyroxine is the drug of choice to treat hypothyroidism. Doses should start low (to avoid cardiovascular side effects) and then be titrated up based on TSH levels.
- All other thyroid hormone formulations (including Thyroid USP, Armour Thyroid, liothyronine, etc.) are NOT recommended for use in hypothyroidism. These are not FDA approved medications and there is no data showing these products are more effective than levothyroxine.
- Generic formulations of levothyroxine are as effective and safe as brand-name Synthroid®. Although several levothyroxine formulations are AB compatible and can be interchanged by a pharmacist, patients should be maintained on the same formulation whenever possible.
References - Dong BJ, Hauck WW, Gambertoglio JG, et al. Bioequivalence of generic and brand-name levothyroxine products in the treatment of hypothyroidism. JAMA. 1997;277(15):1205-1213.
- Rennie D. Thyroid storm. JAMA. 1997;277(15):1238-1243.
- American Thyroid Association. https://www.thyroid.org/
Direct download: 143_-_hypothyroidism.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 18 January 2022
In this episode, we will build up on our previous Weight loss Pharmacotherapy Episode, episode #13 to discuss updates in guidelines, prevention of obesity from comorbidity standpoint, and new treatment agents for weight-loss with a particular focus on Contrave (naltrexone/bupropion), Saxenda (liraglutide), and Wegovy (semaglutide).
Direct download: 142_-_obesity_meds.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 28 December 2021
In this episode, we provide a concise overview of the diagnosis and treatment of hepatorenal syndrome-acute kidney injury (HRS-AKI) with a focus on the new HRS-1 definition (now called HRS-AKI), new data with terlipressin, and the AASLD 2021 guidelines. Key Concepts - At a basic level, HRS-AKI is caused by portal hypertension leading to systemic vasodilation and a prerenal state. Our treatment focuses on increasing vascular volume (usually with albumin) and vasoconstriction to increase renal perfusion.
- The newest HRS-AKI definition borrows most of the AKI definitions from the KDIGO criteria for AKI. HRS-AKI requires cirrhosis, ascites, AKI, and an exclusion of other etiologies of AKI.
- In AKI and HRS-AKI, concentrated (25%) albumin is given. A dose of 1 gm/kg/day (max 100 gm) for two days is used for AKI. For HRS-AKI, a dose of 20-50 grams/day is recommended.
- The preferred vasoconstrictor in HRS-AKI is terlipressin; however, it is not available in the US. Norepinephrine (if in the ICU) is second-line. If not in the ICU, midodrine and octreotide are recommended. Therapy is continued until renal function recovers, if there is no improvement at 4 days, or if a full 14 days of therapy has been given.
References - Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. doi:10.1002/hep.31884
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. doi:10.1016/j.jhep.2018.03.024
Direct download: 141_-_HRS.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 7 December 2021
In this episode, we review the pharmacology, indications, adverse effects, and unique drug characteristics of the most common SSRIs on the market. Key Concepts - SSRIs (selective serotonin reuptake inhibitors) are the drug of choice for depression, anxiety, and a variety of other psychiatric indications.
- Fluoxetine (Prozac) and paroxetine (Paxil) inhibit CYP2D6, a metabolic pathway for several opioid analgesics, tamoxifen, and many other antidepressants.
- Adverse effects of SSRIs start immediately but the beneficial psychiatric effects take up to 1 to 2 months to occur. Patient counseling about the timing of adverse effects and efficacy are important!
- SSRIs should not be abruptly discontinued in patients taking the medication chronically. Withdrawal symptoms can include flu-like symptoms, changes in mood or sleep, and (rarely) even electric-like shocks. To discontinue, the SSRI dose should be tapered down over the period of several weeks.
Direct download: 140_-_ssri.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 16 November 2021
In this episode, we discuss the recent accelerated approval of the new monoclonal antibody-based treatment agent, aducanumab (Aduhelm), by the FDA. We dive into the drug approval process, the efficacy and safety data, and the behind-the-scenes story of the FDA approval. Furthermore, we will present the controversy behind the approval and what it means for stakeholders. Key Concepts - Aducanumab (Aduhelm) is a monoclonal antibody proven to reduce beta amyloid plaques in patients with Alzheimer’s disease.
- Despite reducing plaques, aducanumab did NOT result in meaningful cognitive improvements in patients receiving the drugs versus placebo; additionally, the drug was associated with a potentially concerning adverse effect called ARIA-E (amyloid related imaging abnormalities-edema).
- Despite an advisory panel unanimously recommending AGAINST approval and a lack of clinical outcome improvement, the FDA did go on to approve the drug in patients with Alzheimer’s disease using an accelerated approval process.
- In the episode, we discuss the substantial ramifications of the FDA’s approval primarily in hurting its credibility in validating the safety and efficacy of other medications on the US market.
Direct download: 139_-_aducanumab.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 26 October 2021
In this episode, we discuss the fascinating science of pharmaceutics with Dr. Kristen Ahlschwede and Dr. Rahul Deshmukh. We explore how dosage forms and excipients play an important role in how a drug product behaves in the human body with a particular focus on fentanyl patches, osmotic tablets (with laser-drilled holes), Depakote Sprinkles, and IV amiodarone. Key Concepts - Fentanyl patches were reformulated from a drug-in-a-reservoir system to an adhesive matrix system to prevent abuse and misuse. The new formulation prevents fentanyl from "leaking" out when cut.
- Osmotic tablet systems, such as Procardia XL, Glucotrol XL, and Concerta, use an "active" layer (containing drug) adjacent to a "push" layer that is osmotically active. When the push layer is exposed to water in the GI tract, it swells and pushes the active layer through a small laser-drilled precision hole.
- "Sprinkle" dosage forms typically involve small pellets inside a capsule, such as Depakote Sprinkles. The capsule itself does not delay or extend release; instead, the pellets themselves are involved in prolonging the absorption profile of the drug.
- Amiodarone IV is commercially available in two formulations -- the conventional formulation (Cordarone) contains benzyl alcohol and tween 80 to solubilize the drug but these excipients are associated with hypotension. A newer formulation (Nexterone) uses cyclodextrin as a solubilizing agent and is not associated with hypotension (although has a risk of nephrotoxicity, especially at higher cumulative doses).
Direct download: 138_-_pharmaceutics.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 5 October 2021
In this episode, we reveal what goes on behind the scenes for drug pricing and pharmacy reimbursement with Dr. Benjamin Jolley. Our discussion covers important concepts like PBMs, DIR fees, MAC pricing, and even possible upcoming changes at the federal government. Key Concepts - Prescription drug reimbursement is a major factor in the decline of independent pharmacies nationwide. Complex reimbursement models, fees, and drug pricing structures are frequently not well understood by both patients and many healthcare providers.
- A pharmacy benefits manager (PBM) is a company hired by an insurance company to handle prescription drug coverage and reimbursement. Three PBMs control more than three-quarters of the entire US market and can often dictate the terms of a drug reimbursement contract with pharmacies.
- PBMs determine how much they will pay for the cost of a medication using either a benchmark (such as the average wholesale price minus some percentage) or a list of the maximum allowable cost (MAC) maintained by the PBM. Pharmacies are required to accept the PBM’s reimbursement amount regardless of the cost the pharmacy paid to acquire the drug from a wholesaler.
- DIR fees, clawbacks, and PBM rebate or discount agreements with manufacturers have resulted in lower reimbursements to pharmacies, higher drug prices for patients, and increased profits for PBMs.
Direct download: 137_-_pbm.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 14 September 2021
In this episode, we discuss the recently published major updates in the 9th edition of the anticoagulation guidelines from CHEST. These new recommendations range from initiation of therapy, secondary prevention, and management of post-thrombotic syndrome. Key Concepts - Among patients with cancer-associated VTE, DOACs are preferred over low molecular weight heparins (LMWH) EXCEPT in patients with GI cancers. The preferred anticoagulant in those with GI cancers is either LMWH or apixaban.
- Among patients with antiphospholipid antibody syndrome, warfarin (INR goal 2-3) is preferred over DOAC therapy.
- In the extended phase of treatment (secondary prevention after 3 months of treatment), lower anticoagulant doses should be used (such as apixaban 2.5 mg BID or rivaroxaban 10 mg daily).
- In patients with a DVT, IVC filters should only be used when anticoagulation therapy is contraindicated. IVC filters reduce the risk of PE but do not alter the risk of DVT extension or future DVTs.
- Compression stockings are not recommended for prevention of post-thrombotic syndrome nor for recurrent DVT prevention.
Direct download: 136_-_chest_2021.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 24 August 2021
In this episode, we debunk four medication myths that have persisted for decades: metronidazole and alcohol; statins and hepatotoxicity; cidal vs. static antibiotics; and "sulfa" allergies. Key concepts - Metronidazole does not interact with alcohol (ethanol) and does not cause a disulfiram-like reaction.
- Statins can cause transient increases in liver function tests; however, these increases are not associated with hepatotoxicity. Routine LFT monitoring is not recommended unless clinically indicated signs or symptoms of liver injury exist.
- The distinction of bactericidal versus bacteriostatic antibiotics is irrelevant. No evidence exists showing that having a bactericidal drug has superior efficacy to a bacteriostatic drug.
- A “sulfa” allergy nearly always means an allergy to Bactrim (sulfamethoxazole-trimethoprim). There are many non-antibiotic sulfonamide-containing medications that do not need to be avoided in patients with a sulfa allergy; however, patients with an allergy to any medication have an increased risk of an allergic reaction to other medication classes.
Direct download: 135_-_mythbusters.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 3 August 2021
In this episode, we provide a concise review of the diagnostic criteria and general treatment approach to patients with hypertensive emergencies. Key Concepts - Hypertensive “urgency” is a misnomer - patients do not require immediate therapy and definitely should not receive IV therapy.
- In most cases, the goal blood pressure in hypertensive emergencies is to decrease by no more than 25% in the first hour, achieve a BP of 160/100 in hours 2-6, then over the next 24-48 hours lower to a more normal blood pressure goal.
- Labetalol is the preferred IV push antihypertensive UNLESS patients have acute heart failure, bradycardia, or possibly in patients with asthma/COPD.
- Nicardipine is one of the most commonly used IV infusions for hypertensive emergencies. Most other continuous infusions are reserved for special types of hypertensive emergencies (e.g. nitroglycerin for pulmonary edema or acute MI, esmolol for aortic dissection).
Direct download: 134_-_hypertensive_emergency.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 13 July 2021
In this episode, we will explore depths of COVID 19 vaccine hesitancy - what it is, how to identify and address it, and some helpful resources. Key Concepts - When a patient seems hesitant to consider the COVID-19 vaccine, explore their hesitations further with a simple “why” or “tell more more” question.
- Understand the root of hesitancy and provide personalized responses using motivational interviewing. Key concepts of motivational interviewing include asking open-ending questions, asking the patient to share their concerns, reflective listening, acknowledging without judgement, and asking for permission to share information.
- The goal of a vaccine hesitancy conversation is not necessarily to have the patient receive the vaccine today; the goal is to move the patient one step closer to validated facts (combating misinformation) and consideration of receiving the vaccine.
Direct download: 133_-_vaccine_hesitancy.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 22 June 2021
In this episode, we will explore the history of COVID vaccine development and have a heart-to-heart conversation with Dr. Archana Chatterjee regarding her role as a dean of the RFUMS Chicago Medical School, her career path, and her position and functions on the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC).
Direct download: 132_-_chatterjee.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 1 June 2021
In this episode, we will review the beta-blocker drug class. We discuss their pharmacology, pharmacokinetic/pharmacodynamic parameters, evidence-based use, efficacy, and safety considerations. Key Concepts - Various beta-blockers are divided into four main subtypes: non-selective, B1-selective, beta-blockers with alpha 1 antagonistic activity, and beta-blockers with intrinsic sympathomimetic activity (ISA). These subtypes govern their place in therapy, efficacy, and adverse effects.
- With regards to dosing, “start low and go slow”. The antihypertensive effect is dose-specific, but heart failure therapy requires a GDMT dosing approach to initiate and reach a certain target dose. Do not initiate as a new agent in acutely decompensated heart failure and definitely do not abruptly stop the therapy -- a taper over 1-2 weeks is required.
- Beta blockers are not first-line antihypertensives; however, they should be used in patients with compelling indications, such as systolic heart failure and post-MI. Other uses include angina, atrial fibrillation, migraine, tremors, and more.
- Beta blockers are associated with a number of adverse effects including bradycardia, bronchoconstriction, weight gain, dyslipidemia, hyperkalemia, and masking of hypoglycemia. More severe adverse effects include heart block, exacerbation of heart failure, and morbidity/mortality from acute withdrawal.
Direct download: 131_-_b-blockers.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 11 May 2021
In this episode, we discuss pharmaceutical industry career paths with Dr. Anastasiya Koshkina, a graduate of the RFUMS College of Pharmacy and Associate Director and Clinical Scientist at The Janssen Pharmaceutical Companies of Johnson & Johnson. Key Concepts - When preparing for the fellowship application process, do your homework on the sector(s) you plan to apply for. It is crucial that you have a cohesive and logical story for why you are applying and what makes you a good fit for the position.
- Strong clinical knowledge is important in the pharmaceutical industry even though there are no traditional direct patient care interactions.
- Postgraduate pharmaceutical fellowship programs are looking for applicants with strong academics (high GPA), leadership experience in extracurricular, and research experiences.
- Pharmacy fellowship programs are specific to a sector of a pharmaceutical company (e.g. medical affairs, regulatory affairs, research and development, etc.).. Unlike with pharmacy residencies, fellowships may or may not have rotations; if rotations exist, they are usually many months in length.
- The team (the people you will be working with) are an essential component of a position. It is much better to work with a team that is supportive and committed to your professional development than to have a seemingly perfect job but be part of a team that is not supportive. The people are everything!
Direct download: 130_-_pharma-koshkina.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 20 April 2021
In episode 57, we had in depth discussion of safety issues, general use and recommendations for PPI therapy. In this episode, we briefly review PPI safety concerns and focus on strategies to deprescribe proton pump inhibitors. Key Concepts - PPIs are approved as acid-reducing therapies to treat various conditions related to decreased gastric pH, however, undocumented use of PPI has increased over the years.
- For indicated conditions, PPI should be used at lowest dose for shortest duration possible.
- When used for longer than intended duration, PPIs may cause long-term issues such as hypomagnesemia, low vitamin B12 levels, low iron levels, anemia, C. difficile-associated diarrhea (CDAD), chronic kidney disease (CKD), and hypergastrinemia causing rebound acid hypersecretion.
- Options for limiting PPI use are: abrupt discontinuation, dose reduction, on-demand dosing, slow deprescribing, or switching to alternate therapy. Abrupt discontinuation leads to rebound hypersecretion due to high gastrin levels.
- There is lack of substantial evidence for what deprescribing strategy is better, but decreasing from multiple times a day to single daily dose, reducing the single daily dose, and switching to every other day are all appropriate strategies.
References
Direct download: 129_-_deprescribe_ppi.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 30 March 2021
In this episode, we announce the debut of the HelixTalk Drug Superlative Awards -- awards given to medications on the market that are outstanding or notorious. In announcing these completely fictitious awards, we review key clinical pearls and pitfalls that every clinician should be aware of with these medications. Key Concepts - Drug most likely to be remembered for COVID-19 ineffectiveness rather than its actual FDA indication: hydroxychloroquine
- Commonly used but worst hypertensives on the market: it’s a tie! Atenolol and hydrochlorothiazide.
- Most confusing dosage forms: valproic acid, valproate sodium, and divalproex -- it’s all the same thing!
- Most innovative prodrug: valacyclovir
- Hottest inactive ingredient: it’s a tie! Sulfobutylether-beta-cyclodextrin (SBECD), an excipient in remdesivir, and polyethylene glycol (PEG), an excipient in mRNA vaccines.
Direct download: 128_-_superlative_awards_2021.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 9 March 2021
In this episode, we will discuss basic pharmacology and use of naloxone, available formulations, evidence for its effectiveness, and naloxone-related pharmacy management and regulations. Key Concepts - Naloxone, available in various formulations, is a quick-acting opioid reversal agent approved for use in opioid overdose cases.
- Naloxone access laws are in place in the majority of the states allowing patients and caregivers to obtain the medication as well as administer naloxone.
- These access laws allow pharmacists to dispense naloxone to patients without individual prescriptions under prescriptive authority, standing/protocol orders, or collaborative agreements. Registration, training, education, and reporting requirements may vary per individual state.
- Despite such access laws, overall prescribing of naloxone per high-dose opioid prescriptions remain low. Not many studies are available, but some suggest these laws help reduce opioid overdose deaths and emergency department visits. Implementation and education about such laws need to be improved.
References
Direct download: 127_-_naloxone.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 16 February 2021
In this episode, we chat with Morgan Anderson, PharmD, BCIDP and alumna of RFUMS, about Enterococcal infections including patterns of antimicrobial resistance and recommended treatment options.
Direct download: 126_-_enterococcus.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 26 January 2021
In this episode, we will briefly review the clotting cascade and warfarin's mechanism of action, and then discuss warfarin pharmacogenetics implications and what clinical recommendations and tools are available in order to calculate warfarin dose.
Direct download: 125_-_warfarin_pgx.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 5 January 2021
In this episode, we discuss the FDA·s Emergency Use Authorization (EUA) process and review the resources available to healthcare providers when an EUA for a drug therapy is approved.
Direct download: 124_-_EUA.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 15 December 2020
In this episode, we interview the niece of Dr. Rosalind Franklin, the namesake of our university, who is also named Rosalind Franklin. As part of the university’s centennial celebration of Dr. Franklin, we discuss her amazing life and scientific achievements and reflect on how her story can be applied to our own lives.
Direct download: 123_-_rosalind_franklin100th.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 24 November 2020
In this episode, we discuss how to excel in your next journal club presentation or discussion while avoiding the all-too-common pitfalls for this unique format.
Direct download: 122_-_journal_club.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 3 November 2020
In this episode, we will discuss some of the interesting and unusual warfarin interactions such as acetaminophen, alcohol, agents with antiplatelet properties, non-green vitamin K foods, and CBD/THC.
Direct download: 121_-_warfarin_drug_interactions.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 13 October 2020
In this episode, we discuss the pathophysiology, drug-related causes, diagnosis, and treatment of a common type of hyponatremia called the syndrome of inappropriate antidiuretic hormone (SIADH).
Direct download: 120_-_siadh.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 22 September 2020
In this episode, we discuss the latest dyslipidemia treatment agent bempedoic acid and dive into its pharmacology, use, and clinical evidence.
Direct download: 119_-_bempedoic_acid.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 1 September 2020
In this episode, we discuss the proposed risks of using beta blockers in patients with cocaine use disorders and whether evidence supports this drug interaction as an “absolute” contraindication.
Direct download: 118_-_cocaine_b-blockers.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 11 August 2020
In this episode, we dive into the pharmacology of ACE inhibitors and ARBs, particularly understanding how they assist in preserving renal function in CKD and yet at the same time can harm the kidneys (e.g. cause AKI).
Direct download: 117_-_ckd_and_aki_with_acei-arb.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 21 July 2020
In this episode, we partner with Dr. Danielle Candelario and Dr. Sneha Srivastava, clinical skills faculty at our college, to discuss tips, tricks, and common pitfalls to avoid when performing medication counseling.
Direct download: 116_-_pt_counseling_pearls.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 30 June 2020
In this episode, we discuss the historical significance of Staphylococcus aureus including its patterns of antimicrobial resistance and recommended treatments.
Direct download: 115_-_staph_aureus.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 9 June 2020
In this episode, we discuss important nutritional deficiencies as well as pharmacologic changes that occur in post-bariatric surgery patients.
|
|
Tue, 19 May 2020
In this episode, we review ten clinical pearls about total parenteral nutrition (TPN) that all pharmacists should know.
Direct download: 113_-_tpn_pearls.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 28 April 2020
In this episode, we will discuss the most recent evidence for the use of omega-3 fatty acids (fish oil) for ASCVD risk reduction.
Direct download: 112_-_fish_oil.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 7 April 2020
In this episode, we chat with Eris Tollkuci, PharmD, BCOP regarding what every non-onc healthcare provider should know about immune checkpoint inhibitors (specifically PD-1/PD-L1 inhibitors) in patients with cancer.
Direct download: 111_-_immune_checkpoint_inhibitors.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 17 March 2020
In this episode, we summarize some of the most significant changes in the newest version of American Diabetes Association - 2020 Standards of Medical Care in Diabetes guidelines.
Direct download: 110_-_dm_2020.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 25 February 2020
In this episode, we review the pros, cons, and literature regarding the use of DOACs versus warfarin in obese patients with venous thromboembolism.
Direct download: 109_-_DOACs_obesity.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 4 February 2020
In this episode, we provide an overview of lifestyle medicine, its six pillars, and how it plays a role in improving health outcomes.
Direct download: 108_-_lifestyle_medicine.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 14 January 2020
In this episode, we discuss clinical pearls and management related to chronic and acute hyperkalemia.
Direct download: 107_-_hyperkalemia.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 24 December 2019
In this episode, we discuss the important updates in the 2019 IDSA Community Acquired Pneumonia (CAP) guidelines with Dr. Christie Bertram, PharmD, an Infectious Diseases Clinical Pharmacist and Assistant Professor at Rosalind Franklin University’s College of Pharmacy.
Direct download: 106_-_CAP.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 3 December 2019
In this episode, we review the three oral P2Y12 inhibitors on the market (clopidogrel, prasugrel, ticagrelor) and discuss each agent’s clinical pearls, comparative effectiveness, and unique precautions.
Direct download: 105_-_P2Y12.mp3
Category: general
-- posted at: 6:30am EDT
|
|
Tue, 12 November 2019
In this episode, we will discuss what is the latest and greatest from the GINA (Global INitiative for Asthma) 2019 guidelines, including new recommendations regarding rescue inhalers, tiotropium, and azithromycin for asthma.
Direct download: 104_-_gina2019.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 22 October 2019
In this episode, we discuss the newly published DAPA-HF trial, which studied dapagliflozin (a drug for diabetes) among patients who had heart failure with reduced ejection fraction (HFrEF) many of which did NOT have a history of diabetes.
Direct download: 103_-_dapa-hf.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 1 October 2019
In this episode, we provide an overview of chronic idiopathic constipation and discuss available treatment options with a particular focus on lubiprostone, linaclotide, plecanatide, and prucalopride.
Direct download: 102_-_CIC.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 10 September 2019
In this episode, we will be discussing the recent updates in measles outbreaks, where the vaccination recommendations stand, and other preventative strategies.
Direct download: 101_-_measles.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 20 August 2019
We are proud to announce our 100th HelixTalk episode! In celebration, we sit down with Dr. Marc Abel, the Dean of the College of Pharmacy at RFUMS to discuss the profession of pharmacy and the future of pharmacy education.
Direct download: 100_-_future_of_pharmacy.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 30 July 2019
In this episode, we enlist the help of Associate Dean for Student Affairs, Janeen Winnike, to discuss the various professional pharmacy organizations and the importance of networking via joining at least one as a student or pharmacist.
Direct download: 099_-_pharm_organizations.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 9 July 2019
In this episode, we review guideline recommendations and recent evidence for secondary prevention of stroke, including risk factor modification, anticoagulant therapy (in patients with atrial fibrillation), and antiplatelet therapy (in patients with noncardioembolic stroke).
Direct download: 098_-_secondary_prevention_of_stroke.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 18 June 2019
In this episode, we discuss the latest and the greatest updates regarding aspirin use in primary prevention and provide you with a summary of results from recently published primary literature.
Direct download: 097_-_Aspirin_primary_prevention.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 28 May 2019
In this episode we discuss the recent FDA approval of esketamine (Spravato) for the management of treatment resistant depression as an add on to oral antidepressant therapy. The agent is novel though so, too, may be its adverse effect profile and logistics of administration.
Direct download: 096_-_esketamine.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 7 May 2019
In this episode, we will review the pharmacology, efficacy, and safety of the most common NSAIDs on the market.
Direct download: 095_-_NSAIDs.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 16 April 2019
In this episode, we discuss the changes made in the American Diabetes Association’s 2019 Standards of Diabetes Care guideline as well as updates in therapy recommendations per ADA and ESDA consensus statement published in Fall 2018.
Direct download: 094_-_DM_update_2019.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 26 March 2019
In this episode, we take a deep dive into the use of stimulants and other medications for management of ADHD. After decades of use, technology has resulted in changes to how they are administered. Furthermore new agents have become available, but are they an improvement?
Direct download: 093_-_stimulants_for_ADHD.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 5 March 2019
In this episode, we discuss IV fluids for hospitalized patients, including normal saline (0.9% NaCl) and lactated ringer’s. In addition, we review the newest literature supporting the use of balanced crystalloids over normal saline from the SMART and SALT-ED trials.
Direct download: 092_-_balanced_crystalloids.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 12 February 2019
In this episode, we are excited to have a special guest with us. Dr. Dyson Wake is Senior Clinical Specialist in Pharmacogenomics at NorthShore University HealthSystem’s Center for Molecular Medicine here in Chicagoland and is here to explain the current and future applications of pharmacogenomics to the area of personalized medicine, as well as expose some misconceptions.
Direct download: 091_-_pharmacogenomics.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 22 January 2019
In this episode, we review some of the most important new recommendations from the 2018 ACC/AHA guidelines for the management of blood cholesterol.
Direct download: 090_-_2018_lipid_guidelines.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 1 January 2019
In this episode, we will review the FDA’s Unapproved Drugs Initiative and how it has impacted the availability and pricing of commonly used (but old) medications, such as colchicine and vasopressin.
Direct download: 089_-_Unapproved_Drugs_Initiative.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 11 December 2018
In this episode, we will discuss a new class of medications for preventing migraines called CGRP antagonists, including an overview of their development, clinical efficacy, and future goals of further research in this area.
Direct download: 088_-_CGRP.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 20 November 2018
In this episode, we continue to review insulin therapy including dosing-related specifications, dosing adjustments, injection technique, patient counseling pearls, and the concept of a sliding scale.
Direct download: 087_-_insulins_part_2.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 30 October 2018
In this episode, we discuss a broad overview of insulin topics ranging from the various types of insulins, dosage forms, brand/generic names, pharmacokinetic nuances, injection technique, and a wide variety of clinical pearls.
Direct download: 086_-_insulins_part_1.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 9 October 2018
In this episode, we discuss vaccine related updates with our vaccine expert, Dr. Lauren Angelo. The updates discussed in this episode include the new shingles vaccine, the variety of influenza vaccines on the market, and several other updates.
Direct download: 085_-_vaccine_update.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 18 September 2018
In this episode, we will discuss the advantages and disadvantages of a rate versus rhythm control strategy for atrial fibrillation.
Direct download: 084_-_Rate_vs_rhythm.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 28 August 2018
In this episode, we discuss the importance of accurately recognizing depressive symptoms in patients with kidney disease and provide review the limited available literature regarding treatment in this population. We then discuss guidelines to determine some of the best treatment options for this unique subgroup of patients.
Direct download: 083_-_ESRD_antidepressant.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 7 August 2018
In this episode, we take a closer look at deuteration, specifically involving the medication deutetrabenazine (discussed back in episode 76). We feature a guest contributor from our pharmacy sciences department who assists us as we look at the drug design aspects of this unique molecule and its implications for research moving forward.
Direct download: 082_-_deuteration.mp3
Category: general
-- posted at: 6:00am EDT
|
|


