Wed, 13 December 2023
In this two part episode, we review some of the most important clinical pearls in the pharmacotherapy and practice aspects of hormonal contraceptives with a brief focus on the very first FDA approved OTC hormonal contraceptive product (Opill). Key Concepts (Part 1) - The effectiveness of contraceptives varies based on “ideal use” (e.g. in a clinical trial with optimal compliance) versus “typical use” (e.g. real-world effectiveness in patients who may sometimes be less adherent than in clinical trials). Oral, patch, and ring-based hormonal contraceptives (combination estrogen-progestin or progestin-only formulations) with “typical” use are about ~90% effective, meaning in one year there are ~10 unplanned pregnancies with these contraceptive options.
- When using an estrogen-based oral contraceptive, the estrogen dose should be initiated at a low dose (25 mcg or less per day of ethinyl estradiol). The dose of estrogen may need to be increased if breakthrough bleeding occurs in the early/mid cycle despite being on therapy for at least 6 months.
- Breakthrough bleeding later in the cycle is typically due to an inadequate progestin dose. In general, manufacturers do not provide multiple different formulations with different progestin doses; therefore, if late breakthrough does occur, an alternative formulation with a different progestin should be considered.
- If a patient misses one dose of a combination oral contraceptive, they should take the missed dose as soon as possible (even taking two doses at once if they remember when the next dose is due). If two or more doses are missed, the package insert should be consulted for instructions – management depends on the timing of the cycle, recency of unprotected sex, and other factors.
References
Direct download: 175-hormonal-contraception-part-i.mp3
Category: general
-- posted at: 11:47am EDT
|
|
Mon, 4 December 2023
In this episode, we interview Scott Glosner, PharmD, MPH, BCPS about his extensive experience working at Pfizer in medical outcomes and as a field medical director. Dr. Glosner will share his career journey from a clinical pharmacist transitioning into the pharmaceutical industry in the late 1990s and what current pharmacists and students should know about a job in a pharmaceutical company. Key Concepts - Pharmacists are playing an increasingly important role within the pharmaceutical industry. Prior clinical experience is a significant advantage to applicants for these positions.
- Key characteristics of a competitive pharmacist applicant for an industry position include strong communication skills, being perseverant (“tough skin”), being extremely persistent, and having real-world clinical experience.
- Different companies and job positions within industry often require differing amounts of prior experience. Applicants with more than several years of experience (or equivalent fellowship experience) may be more competitive for positions. Standing out in any way, whether board certification, doing research, networking, etc. is important for any applicant.
- In the future, pharmacists in industry may be playing a greater role in the oncology space, social determinants of health, emerging topics (such as gene therapy), and being capable of analyzing and interpreting “real world” clinical trial data.
Questions for Dr. Scott Glosner? He can be reached at scott.glosner@pfizer.com or on LinkedIn (https://www.linkedin.com/in/scott-glosner-b743234).
Direct download: 174-scott-glosner.mp3
Category: general
-- posted at: 1:22pm EDT
|
|
Tue, 31 October 2023
In this episode, we will discuss the definition of REMS (Risk Evaluation and Mitigation Strategies), why they exist, the role of FDA in administering REMS, types and examples of REMS, and how they impact pharmacy practice. Key Concepts - The REMS (Risk Evaluation and Mitigation Strategies) program was developed in 2007 as part of the FDA’s drug risk management strategies designed to balance risk and benefits of certain drugs.
- Elements of REMS vary depending on the drug, but commonly include medication guides, communication plans, and other elements to assure safe use.
- REMS can require patients, providers, and pharmacies to take certain actions including training, registration, enrollment, safety monitoring, documentation of safety concerns, and follow prescribing and dispensing regulations.
- The FDA captures and assesses data on a regular basis to make changes in the REMS program. It also has authority to enforce compliance and take punitive actions against non-compliant parties.
References
Direct download: 173-REMS.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 10 October 2023
In this episode, we review the role and indications of thrombolytics in acute ischemic stroke. The efficacy, safety, administration considerations, and cost between alteplase and tenecteplase are compared and contrasted. Key Concepts - Alteplase (Activase) is a recombinant DNA version of human TPA (tissue plasminogen activator). Tenecteplase (TNKase) is similar to human TPA except it has three amino acid changes that result in a longer half-life and higher fibrin specificity.
- In patients with stroke, alteplase is given as a bolus followed by a 60-minute infusion. Tenecteplase is given as an IV bolus without the need for an infusion due to its longer half-life.
- Tenecteplase is at least as safe and effective as alteplase in acute ischemic stroke (with some studies showing greater benefit with tenecteplase).
- In patients with acute ischemic stroke who are candidates for mechanical thrombectomy, thrombolytics (with alteplase or tenecteplase) will still be given in patients who meet inclusion criteria and have no exclusion criteria.
References - Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association [published correction appears in Stroke. 2019 Dec;50(12):e440-e441]. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
- Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/NEJMoa1716405
- Kobeissi H, Ghozy S, Turfe B, et al. Tenecteplase vs. alteplase for treatment of acute ischemic stroke: A systematic review and meta-analysis of randomized trials. Front Neurol. 2023;14:1102463. Published 2023 Jan 23. doi:10.3389/fneur.2023.1102463
Direct download: 172-alteplase_vs_tenecteplase.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 19 September 2023
In this episode, we briefly review RSV (respiratory syncytial virus) infections and focus on new data supporting the use of two different RSV vaccines (Abrysvo and Arvexy) in preventing RSV infections in older adults and in pregnant women. Key Concepts - RSV is a contagious respiratory virus that is usually mild and self-limiting in most patients but can cause severe disease especially in young children or older adults with certain risk factors.
- The FDA recently approved two vaccines for RSV (Abrysvo from Pfizer and Arexvy from GSK). The initial FDA approval was for adults 60 years of age and older; however, the FDA recently granted an additional indication for Abrysvo for pregnant women (to prevent the infant from severe RSV infection once born).
- When studied in older adults, both vaccines did meet efficacy criteria but the incidence of RSV infection was relatively low and thus the number needed to treat (NNT) is high. Both studies were done at times with lower RSV prevalence - the NNT would likely be more favorable during RSV outbreaks.
- Unlike Abrysvo, Arvexy (GSK) contains an adjuvant to improve the immune response. Although direct comparisons of efficacy and safety are not appropriate, Arvexy does appear to elicit more systemic adverse effects such as fever, myalgias, headache, and fatigue.
References - Respiratory Syncytial Virus Infection (RSV). Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/index.html
- Abrysvo (respiratory syncytial virus vaccine). US Food & Drug Administration. https://www.fda.gov/vaccines-blood-biologics/abrysvo
- Arexvy (respiratory syncytial virus vaccine, adjuvanted). US Food & Drug Administration. https://www.fda.gov/vaccines-blood-biologics/arexvy
- Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023. Morbidity and Mortality Weekly Report (MMWR). July 21, 2023 / 72(29);793-801. https://www.cdc.gov/mmwr/volumes/72/wr/mm7229a4.htm
- CDC. ACIP Recommendations. Last reviewed August 4, 2023. www.cdc.gov/vaccines/acip/recommendations.html. Accessed August 23, 2023.
- RENOIR - Walsh EE, Pérez Marc G, Zareba AM, et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
- AReSVi-006 - Papi A, Ison MG, Langley JM, et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N Engl J Med. 2023;388(7):595-608. doi:10.1056/NEJMoa2209604
- MATISSE - Kampmann B, Madhi SA, Munjal I, et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023;388(16):1451-1464. doi:10.1056/NEJMoa2216480
- RSV-NET Interactive Dashboard. Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/research/rsv-net/dashboard.html
- ACIP Meeting Information - Meeting Materials. https://www.cdc.gov/vaccines/acip/meetings/index.html
Direct download: 171-rsv.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 29 August 2023
In this episode, together with our faculty colleague, Dr. Roberta Dume, PharmD, BCPP, we discuss the pharmacologic options and evidence for the treatment of opioid use disorder (OUD) and how pharmacists play a vital role in assisting patients suffering from opioid use disorder. Key Concepts - The treatment for OUD should be provided by either the treating clinician or a certified Opioid Treatment Provider (OTP) using one of three FDA-approved therapies which include buprenorphine, methadone, and naltrexone.
- Selection of the OUD treatment depends on availability of treatment provider; pharmacologic agent specific factors such as efficacy, dose titration, safety, and need for detoxification; and patient factors such as ability to safe-keep medications, adherence to required clinic visits, or presence of comorbidities.
- Pharmacists can play an important role for patients needing OUD by providing treatment education, treatment induction, monitoring treatment outcomes, harm reduction by providing naloxone and related education, and utilizing preventative strategies such as monitoring opioid use, offering non-opioid pain management options, and promoting safe storage and disposal of opioids.
References
Direct download: 170-opioid-use-disorder.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 8 August 2023
In this episode, we announce the second iteration of the HelixTalk Drug Superlative Awards -- awards given to medications on the market that are outstanding or notorious. In announcing these completely fictitious awards, we review key clinical pearls and pitfalls that every clinician should be aware of with these notable medications. Key Concepts - The award for the most unique phase III patient population for a widely used medication goes to … Pneumovax-23 (PPSV-23) for its predecessor versions that were studied in South African novice gold miners.
- The award for the most misunderstood boxed warning goes to … all of the DOACs (but specifically apixaban and rivaroxaban). In particular, due to BOTH an increased risk of thrombosis and bleeding when switching from a DOAC to warfarin therapy in patients with atrial fibrillation.
- The award for the biggest difference between pharmacokinetic properties and pharmacodynamic effects goes to … aspirin due to its short-half life and short duration of analgesic effect and yet very prolonged antiplatelet effect.
- The award for the drug that should be dispensed with extra toilet paper … TIE between irinotecan and clindamycin. The most common dose-limiting adverse effect of irinotecan is diarrhea – loperamide is extensively used in these patients. Clindamycin earns the award because it is the antibiotic most associated with Clostridium difficile-associated diarrhea (CDAD).
References - Smit P, Oberholzer D, Hayden-Smith S, Koornhof HJ, Hilleman MR. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA. 1977;238(24):2613-2616.
Farrar JL, Childs L, Ouattara M, et al. Systematic Review and Meta-Analysis of the Efficacy and - Effectiveness of Pneumococcal Vaccines in Adults. Pathogens. 2023;12(5):732. Published 2023 May 19. doi:10.3390/pathogens12050732
- Pavia M, Bianco A, Nobile CG, Marinelli P, Angelillo IF. Efficacy of pneumococcal vaccination in children younger than 24 months: a meta-analysis. Pediatrics. 2009;123(6):e1103-e1110. doi:10.1542/peds.2008-3422
- Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Sferra TJ, Hernandez AV, Donskey CJ. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013 Sep;68(9):1951-61. doi: 10.1093/jac/dkt129.
Direct download: 169-superlatives-2023.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 18 July 2023
There has been a lot of news about abortion (abortifacient) medications recently. Since the overturn of Roe v. Wade in 2022, individual states passed their own laws restricting access to abortion, this includes access to abortion medications. This clearly impacts the way pharmacists practice. In this episode, we summarize the science behind the two main abortive drugs, mifepristone and misoprostol, and provide a picture of how the access to these medications stand in the United States. Key Concepts - Among other modalities to terminate pregnancies, medication abortion is a safe and alternative option that is picking up popularity given recent changes post-Dobbs vs. Jackson WHO decision.
- The FDA-approved use of combination mifepristone and misoprostol regimen to terminate pregnancy up to 70 days (10 weeks of gestation) is based on strong evidence for its efficacy and safety.
- Since the overturning of Roe vs. Wade in 2022, states have taken their own action to further restrict or increase access to abortion services including access to medication abortion.
- These legal changes further impact dispensing of mifepristone and misoprostol by pharmacists across the country adding to more confusion. Legal councils, state boards of pharmacies, or state pharmacy associations may serve as suitable resources to consult regarding these fast-changing laws.
References
Direct download: 168-medication-abortion.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 27 June 2023
In this episode, we review the science behind genetic differences in humans in the CYP2D6 hepatic enzyme responsible for drug metabolism and how these genetic variants can lead to certain drugs being metabolized far too much or far too little, which can cause drug toxicities or a lack of effectiveness. Key Concepts - About 20-25% of drugs on the market are metabolized by CYP2D6. Humans have a huge degree of variability in CYP2D6 metabolism ranging from “ultra” metabolizers to “poor” metabolizers.
- Drugs that heavily rely on CYP2D6 metabolism are prone to large variability in responses due to these genetic differences. Some drugs rely on metabolic inactivation of CYP2D6 whereas other drugs use the enzyme to become converted to a more active compound.
- Codeine and tramadol both heavily rely on CYP2D6 activation to a more potent opioid compound. Patients with excessive CYP2D6 activity will have toxicities (from too much of an active metabolite) whereas patients with low CYP2D6 activity will have little therapeutic effect.
- Numerous antidepressants (paroxetine, nearly all tricyclic antidepressants, and venlafaxine) rely on CYP2D6 metabolism. Differences in CYP2D6 metabolism have been shown to either cause toxicity or a lack of effectiveness with these medications.
References - Chartrand R, Forte AM, Hoger JD, Kane SP, Kisor DF. Pharmacogenomics and Commonly Prescribed Medications. AdvanCE. October 10, 2022. https://www.advancepharmacist.com/courses/pharmacogenomics-and-commonly-prescribed-medications.
- Caudle KE, Sangkuhl K, Whirl-Carrillo M, et al. Standardizing CYP2D6 Genotype to Phenotype Translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin Transl Sci. 2020;13(1):116-124. doi:10.1111/cts.12692
- Bousman CA, Stevenson JM, Ramsey LB, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants [published online ahead of print, 2023 Apr 9]. Clin Pharmacol Ther. 2023;10.1002/cpt.2903. doi:10.1002/cpt.2903
- Crews KR, Monte AA, Huddart R, et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin Pharmacol Ther. 2021;110(4):888-896. doi:10.1002/cpt.2149
Direct download: 167-pgx-of-2d6.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 6 June 2023
In this episode, we compare hydrochlorothiazide and chlorthalidone, but specifically from a cardiovascular outcomes perspective when used in patients with hypertension. Key Concepts - Chlorthalidone, hydrochlorothiazide, and indapamide are available thiazide diuretics for treatment of hypertension; however, hydrochlorothiazide is the most commonly used agent.
- Chlorthalidone is more potent in reducing blood pressure but also is associated with a higher risk of electrolyte abnormalities compared to HCTZ.
- Recent studies for cardiovascular outcomes show that chlorthalidone is not better than HCTZ in preventing CV outcomes, but increases risk for hypokalemia, need for monitoring and even potassium supplementation.
References
Direct download: 166-thiazide-throwdown.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 16 May 2023
In this episode, we discuss the concerns of QTc prolongation, which can cause a fatal arrhythmia called torsades de pointes (TdP). We cover the difference between QT and QTc, how to interpret a QTc (and when it is inaccurate), common medications that prolong QTc, and how pharmacists can evaluate the risk of QTc/TdP in patients who are receiving QTc-prolonging therapies. Key Concepts - The QTc interval is the QT interval that has been “corrected” for heart rate. In nearly all cases, when describing a QT interval, it should be expressed as the QTc.
- Although a prolonged QTc is usually defined as a QTc exceeding 450-480 msec, the risk of torsades de pointes (TdP) begins to become concerning when the QTc is more than 500 msec, 15-20% longer than baseline, or if the QTc has increased by more than 60 msec.
- Vaughan-Williams Class III antiarrhythmics are most implicated in QTc prolongation and TdP risk. These therapies include sotalol, dofetilide, and dronedarone. Although amiodarone is a class III antiarrhythmic, its risk of TdP is quite low despite the fact that it often substantially prolongs the QTc.
- When pharmacists are assessing the risk of QTc prolongation and TdP, multiple factors (not just the QTc itself) should be considered. Risk scores, like the Tisdale Risk Score, as well as considering the risks/benefits of switching drug therapy, should be evaluated.
References
Direct download: 165-qtc.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 25 April 2023
In this episode, we will discuss the most important updates from the American Diabetes Association’s 2023 Standards of Care in Diabetes. Key Concepts - The first-line therapy for type II diabetes is based on whether the primary goal of therapy is cardiorenal benefit (reduced risk of ASCVD, heart failure, or CKD) or glycemic and weight goals.
- For cardiorenal benefit, GLP1 receptor agonists and SGLT2 inhibitors are heavily emphasized. For glycemic control and weight gain, GLP1 receptor agonists (or GLP1/GIP in the case of tirzepatide) have a very favorable effect on weight loss and glycemic control. While metformin is still mentioned, it is no longer the sole, first-line therapy for type II diabetes.
- For patients with diabetes and a high risk of ASCVD (20% or higher), high-intensity statins, ezetimibe, and/or PCSK9 inhibitors are recommended to achieve an LDL less than 70 mg/dL. In patients with a history of ASCVD events, these same therapies are used to achieve a recommended LDL goal of less than 55 mg/dL.
- Among selected patients with diabetes and CKD with albuminuria, finerenone (a new mineralocorticoid receptor antagonist) is recommended to improve renal and cardiovascular outcomes.
- A variety of different therapies are now recommended for neuropathic pain, including gabapentinoids, SNRIs, TCAs, and several antiseizure medications (lamotrigine, lacosamide, oxcarbazepine, and valproic acid).
- A wide variety of other new recommendations are discussed in the episode, including NASH/NAFLD, obesity and weight management, special populations, diabetes technology, and health behavior changes.
References
Direct download: 164-diabetes-2023.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 4 April 2023
In this episode, we review clinical pearls and common pitfalls of immunosuppression regimens for organ transplantation with a particular focus on tacrolimus and mycophenolate. Key Concepts - Most recipients of an organ transplantation will be on a two or three drug regimen. The most common regimen is tacrolimus and mycophenolate with/without a corticosteroid.
- Tacrolimus is hepatically eliminated and susceptible to CYP3A4 and PGP drug interactions. Particularly at higher drug concentrations, it is associated with nephrotoxicity and neurotoxicity (among several other adverse effects).
- Mycophenolate is unstable in the acidic environment of the stomach. The two formulations on the market are CellCept (which uses a prodrug, mycophenolate mofetil, that is converted in the liver to an active compound) and Myfortic (an enteric-coated formulation of mycophenolic acid, which releases after exiting the stomach).
- The intensity of an immunosuppression regimen is determined by numerous factors, including the type of organ, how long ago the organ was transplanted, if acute rejection has occurred in the past, patient-specific risk factors, and more.
Additional Resources - Register to be a donor at Donate Life America (https://donatelife.net) or at the HRSA OrganDonor.gov site (https://www.organdonor.gov)
- Learn more about stem cell donation and transplant at https://bethematch.org
Direct download: 163-transplant-tango.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 14 March 2023
In this first ever CE episode, we discuss the A-Zs of continuous glucose monitors (CGMs). In specific, our learning objective for the CE are: -
Describe commonly available types of continuous glucose monitors (CGMs) in the US market and the features and capabilities of these devices. -
Summarize the evidence and guideline recommendations for use of CGMs in the management of diabetes. -
Identify the role of the pharmacist in the selection of CGMs and provision of education to patients and providers. -
Interpret the ambulatory glucose profile (CGM data output) and recommend changes in antihyperglycemic regimen for a patient. ACPE-Accredited Pharmacist CE (1.0 hrs) To obtain CE credit for a $5 fee, visit the following link: https://rfums.wufoo.com/forms/z1qzh5vf0ggr832/. Once payment is successful, you will be redirected to our CE partner (CE Impact) to complete an evaluation and to earn 1.0 hour of CE credit. CE is available for 12 months after episode publication. Key Concepts - There are two main types of stand-alone personal CGMs available in the US market – real-time (rtCGM) and intermittently scanning (isCGM). [1] These CGMs vary in their features such as sensor wear time, sensor warm up time, sensor application site, reader availability, approved age for use, fingerstick calibration, non-adjunctive FDA labeling, interconnectability with other technology such as insulin pumps, and drug interactions – these variabilities can be used in decision-making when selecting an appropriate CGM for a patient. [2-7]
- Based on the evidence for use, both types of CGMs (real-time and intermittently scanning) are recommended in patients with Type 1 and Type 2 diabetes who are on multiple-daily insulin or continuous insulin infusion (pump), patients with Type 2 diabetes on basal insulin therapy, and as adjunct use in patients with diabetes who are pregnant. The strength of recommendations in general is stronger for real-time CGMs than for intermittently scanning CGMs. [1,11] These recommendations are supported by the evidence that CGMs can help improve glucose control, reduce risk of hypoglycemia, diabetes-related hospitalizations, and patient/caregiver satisfaction.
- Pharmacists play an integral role in education, on-going support, data interpretation, and resulting disease management in patients who qualify for CGM use and providers who care for patients with diabetes. [14]
- The ambulatory glucose profile is a standardized data output that informs understanding of glucose trends. [15] The recommended goal for most patients is to maintain a glucose range between 70-180 mg/dL with at least 70% of time spent in this range with variability coefficient of no more than 36%. [1,11,15]
Supplemental Content Comparison of rtCGM and isCGM devices 
"Mary's" Example AGP Report (adapted from Battelino et al.) 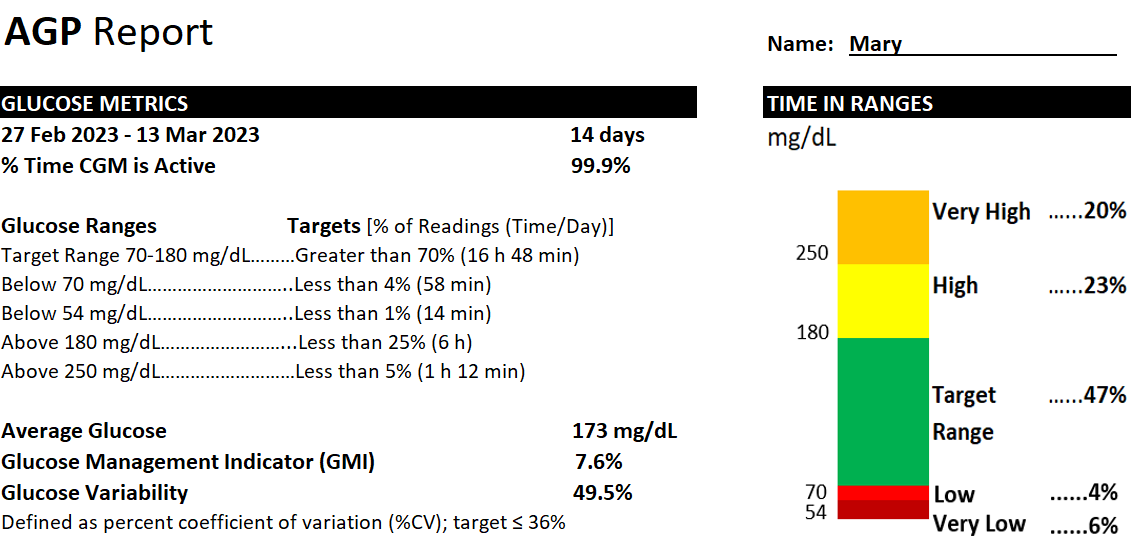 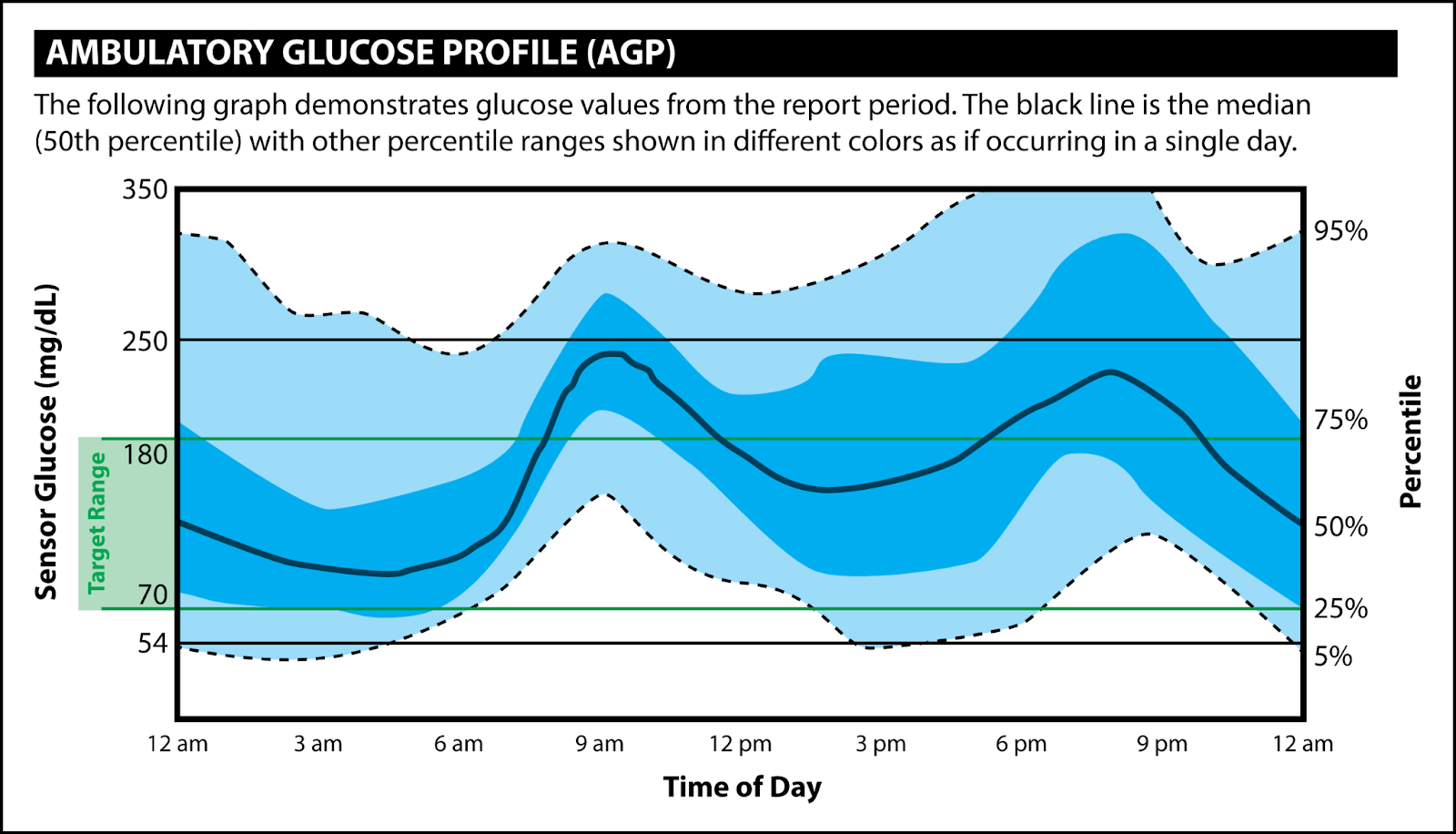
References - ElSayed NA, Aleppo G, Aroda VR, et al. American Diabetes Association. Chapter 7. Diabetes technology: Standards of medical care in diabetes - 2023. Diabetes Care. 2023;46(suppl 1):S111-S127.
- Dexcom G6 User Guide. Dexcom, Inc. 2020. Accessed February 20, 2023. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf.
- Dexcom G7 User Guide. Dexcom, Inc. 2022. Accessed February 20, 2023. https://dexcompdf.s3.us-west-2.amazonaws.com/en-us/G7-CGM-Users-Guide.pdf#page=12
- Guardian Connect System User Guide. Medtronic MiniMed. 2020. Accessed February 20, 2023. https://www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf.
- Eversense E3 User Guide. Sensionics, Inc. 2022. Accessed February 20, 2023. https://www.eversensediabetes.com/wp-content/uploads/LBL-4002-01-001-Rev-F_Eversense-E3-User-Guide_mgdL_R1_web.pdf
- FreeStyle Libre 3 User’s Manual. Abbott Diabetes Care Inc. 2022. Accessed February 20, 2023. https://freestyleserver.com/Payloads/IFU/2022/q2/ART44140-002_rev-A.pdf
- FreeStyle Libre 2 User’s Manual. Abbott Diabetes Care Inc. 2020. Accessed February 20, 2023. https://freestyleserver.com/Payloads/IFU/2020/q2/ART40703-001_rev-D-Web.pdf.
- Products. American Diabetes Association. Accessed February 20, 2023. https://consumerguide.diabetes.org/
- Wood A, O'Neal D, Furler J, Ekinci EI. Continuous glucose monitoring: a review of the evidence, opportunities for future use and ongoing challenges. Intern Med J. 2018 May;48(5):499-508.
- Edelman SV, Argento NB, Petty SJ, Hirsch IB. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care. 2018;41:2265-2274.
- Fonseca VA, Grunberger G, Anhalt H, et al. Continuous glucose monitoring: A consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22(8):1008-21.
- Reiterer F, Polterauer P, Schoemaker M, Schmelzeisen-Redecker G, Freckmann G, Heinemann L, Del Re L. Significance and Reliability of MARD for the Accuracy of CGM Systems. J Diabetes Sci Technol. 2017 Jan;11(1):59-67. doi: 10.1177/1932296816662047. Epub 2016 Sep 25. PMID: 27566735; PMCID: PMC5375072.
- Food and Drug Administration. Premarket Notification 510(k). 2022. Accessed February 25, 2023. https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k.
- Isaacs, Diana. The pharmacist’s role in continuous glucose monitoring. Pharmacy Today. 2020;26:37-54.
- Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603.
Direct download: 163-cgm.mp3
Category: general
-- posted at: 7:00am EDT
|
|
Tue, 21 February 2023
In this episode, we interview Dr. Shannon Rotolo and Dr. Alex Berce regarding Illinois and Wisconsin drug repository programs – these are programs that allow certain medications to be donated to participating sites and then redistributed to patients at a very low dispensing cost. Key Concepts - Drug repository programs allow participating sites to accept certain donated medications and redistribute these medications to needy patients at a very low dispensing cost.
- Drug repository programs are regulated by state law and the specifics of the process do vary by state. In Illinois and Wisconsin, donated medications must be in their original containers with tamper-evident packaging, cannot be controlled substances, and must have a 90-day expiration window at the time of donation.
- Pharmacists can play an important role in advocating for patients and the profession of pharmacy. The involvement of pharmacists in legislation is critical to make sure that new laws are actually “functional” and can achieve their intended purpose.
References For additional information about our guests, contact Dr. Shannon Rotolo at Shannon.Rotolo@uchospitals.edu or Dr. Alex Berce at alex@goodvaluerx.com.
Direct download: 161-drug-repositories.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 31 January 2023
In this episode, we discuss the evidence, safety, and place in therapy of Auvelity® (dextromethorphan-bupropion), a newly approved antidepressant with a unique mechanism of action and interesting pharmacokinetic considerations. Key Concepts - Auvelity® (bupropion-dextromethorphan) was FDA approved in 2022 for major depressive disorder (MDD). The bupropion component inhibits CYP2D6 metabolism and increases serum concentrations of dextromethorphan. The proposed mechanism of benefit in MDD is via dextromethorphan (as an NMDA antagonist) and possibly with bupropion (as a dopamine/norepinephrine reuptake inhibitor).
- Although the bupropion component in Auvelity® is being used for its drug interaction, the dose is a therapeutic dose and carries several warnings and precautions, including the risk of seizure and hypertension.
- In short (6-week) clinical trials, Auvelity® improved depression symptoms quickly (within 1-2 weeks), which is faster than many other antidepressants. Auvelity® is associated with dizziness, anxiety, hyperhidrosis, nausea, headache, diarrhea, and dry mouth.
- As a CYP2D6 inhibitor, the bupropion component of Auvelity® will cause drug interactions with many other medications, including some antidepressants, antipsychotics, and opioid analgesics (among others).
References - Iosifescu DV, Jones A, O'Gorman C, et al. Efficacy and Safety of AXS-05 (Dextromethorphan-Bupropion) in Patients With Major Depressive Disorder: A Phase 3 Randomized Clinical Trial (GEMINI). J Clin Psychiatry. 2022;83(4):21m14345. Published 2022 May 30. doi:10.4088/JCP.21m14345
- Tabuteau H, Jones A, Anderson A, Jacobson M, Iosifescu DV. Effect of AXS-05 (Dextromethorphan-Bupropion) in Major Depressive Disorder: A Randomized Double-Blind Controlled Trial. Am J Psychiatry. 2022;179(7):490-499. doi:10.1176/appi.ajp.21080800
- Kotlyar M, Brauer LH, Tracy TS, et al. Inhibition of CYP2D6 activity by bupropion. J Clin Psychopharmacol. 2005;25(3):226-229. doi:10.1097/01.jcp.0000162805.46453.e3
Direct download: 160-auvelity.mp3
Category: general
-- posted at: 6:00am EDT
|
|
Tue, 10 January 2023
In this episode, we highlight important changes to the 2023 GOLD Guidelines for COPD. In particular, we discuss a revision to the GOLD group classification system and the preferred initial therapies in patients with COPD. Key Concepts - The newest GOLD COPD guidelines now recognize three GOLD groups – “A”, “B”, and “E”. Group “E” (formerly groups C and D) are patients with frequent exacerbations (defined as 2 or more in the past 12 months or 1 exacerbation requiring hospitalization).
- For group “E” patients, the preferred initial inhaler regimen is a LABA+LAMA. Triple therapy (LABA+LAMA+ICS) can be considered if blood eosinophils are elevated.
- “Triple therapy” (LABA+LAMA+ICS) has gained traction based on the IMPACT and ETHOS trials – this regimen reduced exacerbations and mortality compared to LABA+LAMA and LABA+ICS.
- With an exploding market of new COPD inhalers, the role of the pharmacist is even more critical to help identify affordable medications and provide patient education for proper inhaler technique.
References - Global Strategy for Prevention, Diagnosis, and Management of COPD: 2023 Report. Global Initiative for Chronic Obstructive Lung Disease (GOLD). https://goldcopd.org/2023-gold-report-2/
- IMPACT study: Lipson DA, Barnhart F, Brealey N, et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N Engl J Med. 2018;378(18):1671-1680. doi:10.1056/NEJMoa1713901
- ETHOS study: Rabe KF, Martinez FJ, Ferguson GT, et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N Engl J Med. 2020;383(1):35-48. doi:10.1056/NEJMoa1916046
Direct download: 159-gold-2023.mp3
Category: general
-- posted at: 6:00am EDT
|
|


